2011.0.00510.S/2011.0.00510.S_2012-02-24/2011.0.00510.S/sg_ouss_id/group_ouss_id/member_ouss_id1/calibrated
full summary of this dataset can be found here
Spectral setup
| SpwID | Frame | #chan | skyfreq(MHz) | Chwid(kHz) | BW(MHz) | beam("x" deg) |
|---|---|---|---|---|---|---|
| 0 | TOPO | 3840 | 113625.2 ~ 115499.8 | 488.281 | 1875.000000 | 3.4 x 2.5, -36.2 |
| 1 | TOPO | 3840 | 111750.2 ~ 113624.8 | 488.281 | 1875.000000 | 3.5 x 2.5, -36.3 |
| 2 | TOPO | 3840 | 101625.2 ~ 103499.8 | 488.281 | 1875.000000 | 3.8 x 2.8, -35.7 |
| 3 | TOPO | 3840 | 99750.2 ~ 101624.8 | 488.281 | 1875.000000 | 3.9 x 2.9, -36.8 |
Observed fields
| FieldID | FieldName | R.A. | Dec | Epoch | nRows |
|---|---|---|---|---|---|
| 0 | J1329-5608 | 13:29:01.144927 | -56.08.02.66571 | J2000 | 115080 |
| 2 | Mars | 10:43:42.059512 | +10.22.48.92654 | J2000 | 21000 |
| 4 | Boomerang_Nebula | 12:44:43.188420 | -54.31.22.27470 | J2000 | 65760 |
| 5 | Boomerang_Nebula | 12:44:43.235700 | -54.30.59.78833 | J2000 | 57540 |
| 6 | Boomerang_Nebula | 12:44:45.401520 | -54.31.33.87437 | J2000 | 57540 |
| 7 | Boomerang_Nebula | 12:44:45.448800 | -54.31.11.38800 | J2000 | 57540 |
| 8 | Boomerang_Nebula | 12:44:45.496080 | -54.30.48.90163 | J2000 | 57540 |
| 9 | Boomerang_Nebula | 12:44:47.661900 | -54.31.22.98767 | J2000 | 57540 |
| 10 | Boomerang_Nebula | 12:44:47.709180 | -54.31.00.50130 | J2000 | 57540 |
| 11 | Mars | 11:27:03.174714 | +06.43.46.93651 | J2000 | 30600 |
| 12 | Mars | 11:27:06.848536 | +06.43.33.41277 | J2000 | 30600 |
Spectral window: 0
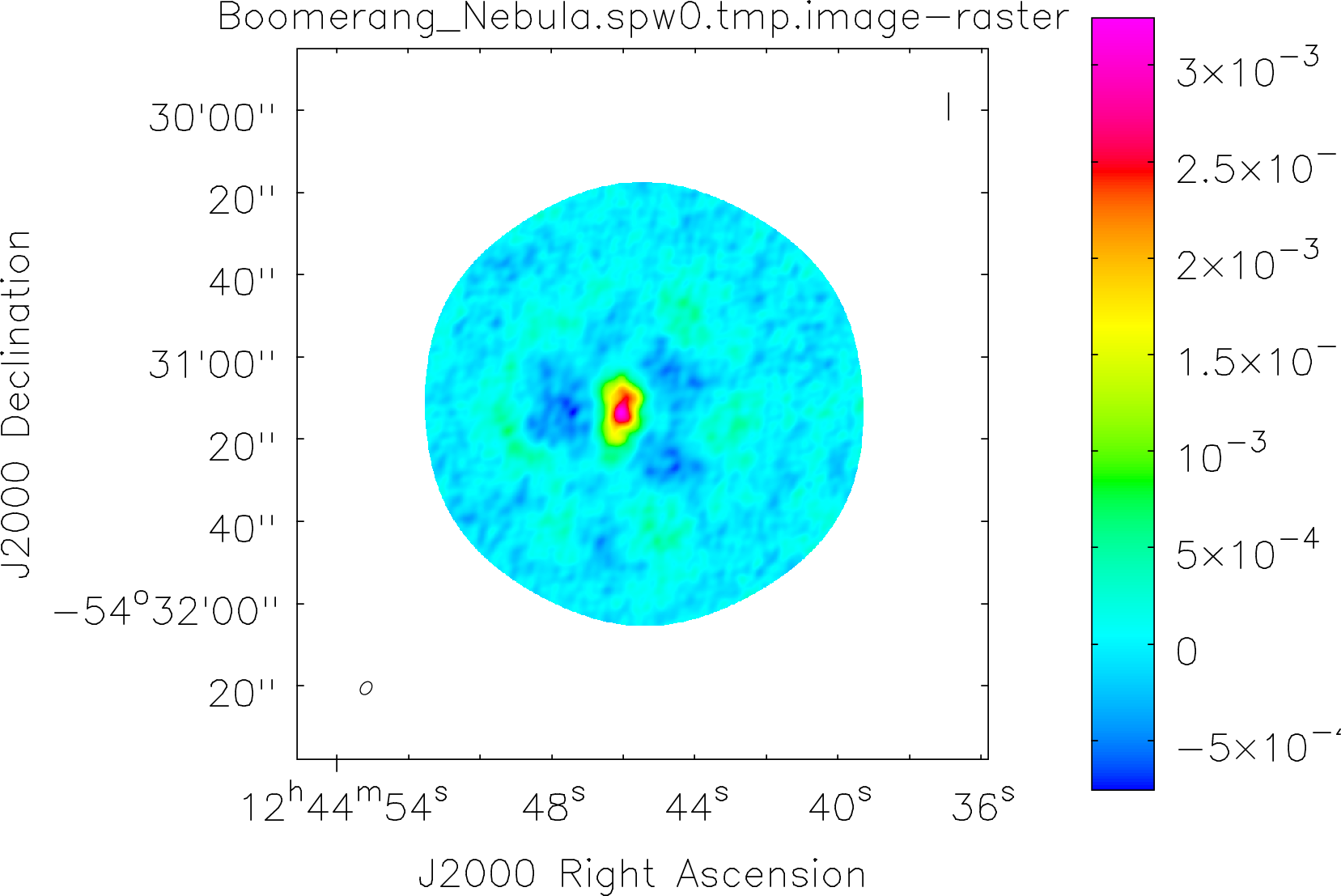
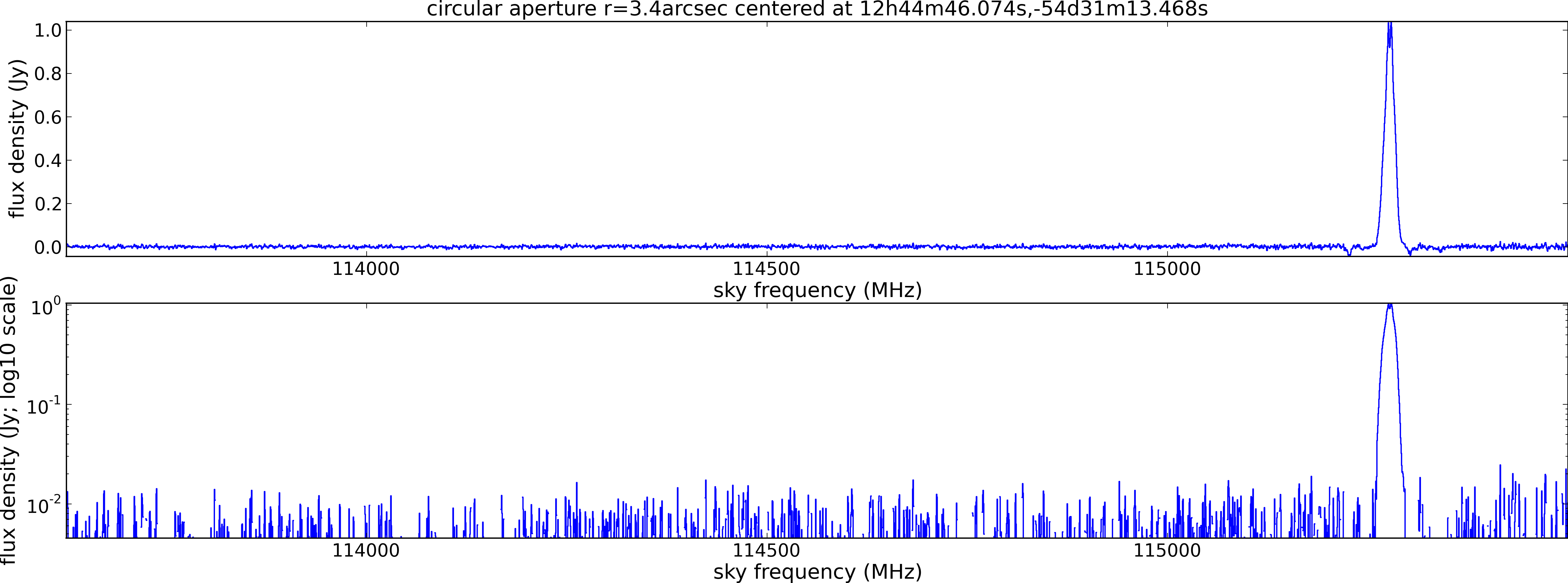
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 113641.053 | 13CH3CH2CN 13(5,9)-12(5,8) |
| 113641.142 | 13CH3CH2CN 13(5,8)-12(5,7) |
| 113657.635 | CH2CHCN 12(2,11)-11(2,10) |
| 113691.460 | 13CH3CH2CN 13(4,10)-12(4,9) |
| 113693.000 | unidentified |
| 113695.000 | unidentified |
| 113697.367 | 13CH3CH2CN 13(4,9)-12(4,8) |
| 113729.000 | unidentified |
| 113743.007 | CH3OCHO 9(3,6)-8(3,5) E |
| 113756.646 | CH3OCHO 9(3,6)-8(3,5) A |
| 113766.420 | HCCCHO 12(1,11)-11(1,10) |
| 113818.000 | unidentified |
| 113820.120 | 29SiC2 5(0,5)-4(0,4) |
| 113831.151 | CH2CHCN 18(2,16)-18(1,17) |
| 113844.000 | unidentified |
| 113978.248 | CH3CH2CN 13(0,13)-12(0,12) |
| 114003.831 | CH3OCH3 18(2,16)-18(1,17) AE+EA |
| 114005.399 | CH3OCH3 18(2,16)-18(1,17) EE |
| 114006.968 | CH3OCH3 18(2,16)-18(1,17) AA |
| 114064.848 | t-CH3CH2OH 2(2,0)-1(1,1) |
| 114092.612 | 18OCS 10-9 |
| 114113.000 | unidentified |
| 114182.510 | C4H 12-11 J=25/2-23/2 |
| 114221.040 | C4H 12-11 J=23/2-21/2 |
| 114291.000 | unidentified |
| 114313.000 | unidentified |
| 114336.000 | unidentified |
| 114362.210 | 30SiC2 5(2,4)-4(2,3) |
| 114443.946 | t-CH3CH2OH 17(2,16)-16(3,13) |
| 114485.037 | HC5N 43-42 |
| 114565.381 | SO2 29(3,27)-28(4,24) |
| 114574.438 | 34SO2 6(3,3)-7(2,6) |
| 114615.021 | H13CCCN 13-12 |
| 114621.567 | CH2CHCN 12(2,10)-11(2,9) |
| 114650.932 | CH3OH 18(-2,17)-18(1,17) E |
| 114737.170 | C4H 2S J=12-11 2v7 L |
| 114793.820 | C4H 2S J=12-11 2v7 U |
| 114831.084 | HCCC15N 13-12 |
| 114840.000 | unidentified |
| 114861.000 | unidentified |
| 114888.234 | CH3OCHO 23(6,18)-22(7,15) A |
| 114897.372 | c-H13CCCH 3(0,3)-2(1,2) |
| 114940.177 | CH3CHO 6(0,6) - 5(0,5) E |
| 114959.909 | CH3CHO 6(0,6) - 5(0,5) A++ |
| 115021.000 | unidentified |
| 115038.874 | C6H 23/2 J=83/2-81/2 e |
| 115072.307 | CH3OCH3 9(2,8)-9(1,9) AE+EA |
| 115075.086 | CH3OCH3 9(2,8)-9(1,9) EE |
| 115077.864 | CH3OCH3 9(2,8)-9(1,9) AA |
| 115083.236 | C6H 23/2 J=83/2-81/2 f |
| 115141.000 | unidentified |
| 115153.935 | NS J=5/2-3/2 F=7/2-5/2 e |
| 115156.812 | NS J=5/2-3/2 F=5/2-3/2 e |
| 115185.411 | NS J=5/2-3/2 F=3/2-3/2 e |
| 115212.000 | unidentified |
| 115216.800 | C4H 23/2 J=25/2-23/2 1v7f |
| 115271.202 | CO 1-0 |
| 115382.375 | SiC2 5(0,5)-4(0,4) |
| 115455.898 | CCCO 12-11 |
Spectral window: 1
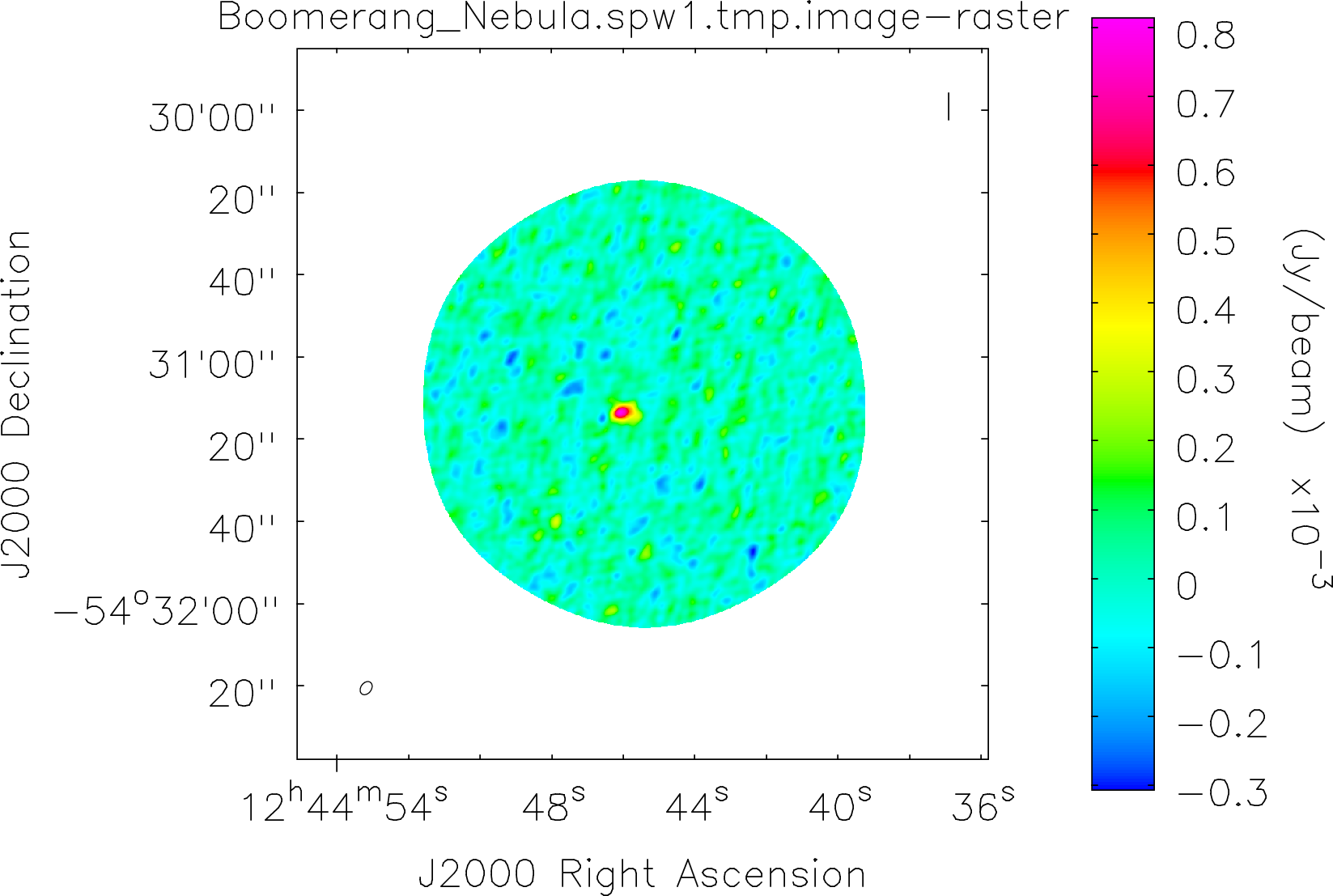
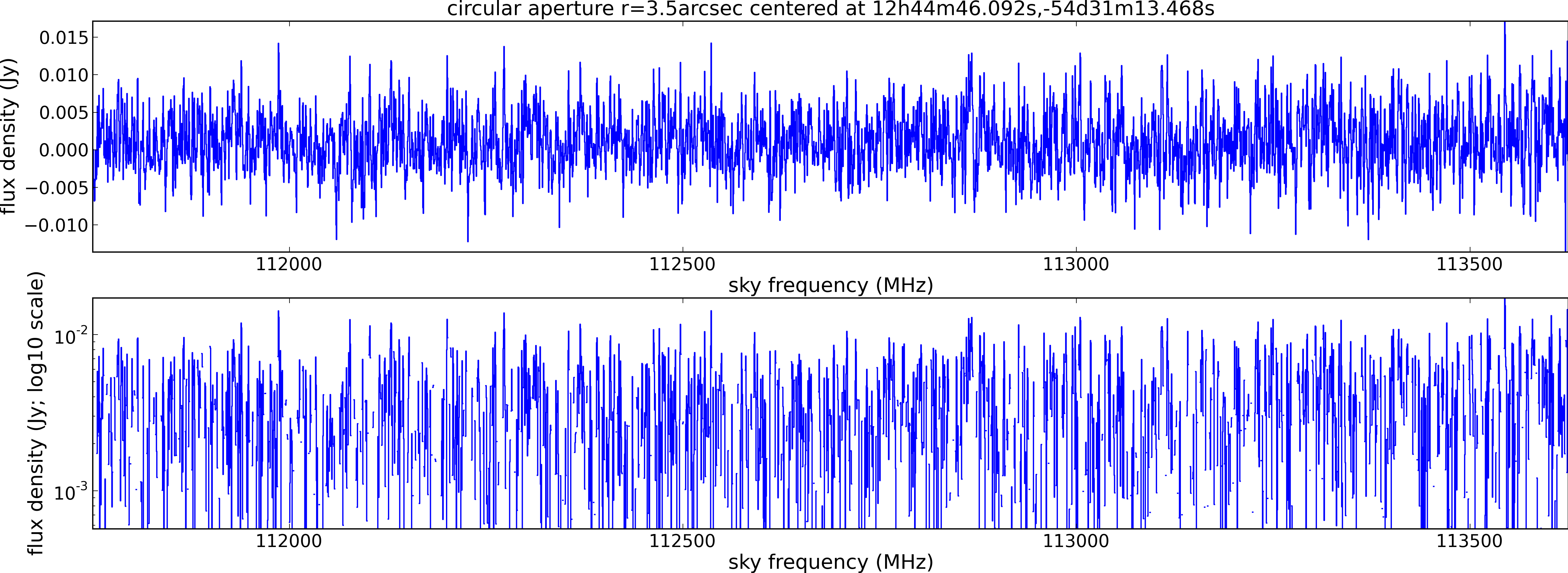
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 111755.028 | SO2 31(3,29)-30(4,26) |
| 111782.596 | CH3OCH3 7(0,7)-6(1,6) AA |
| 111783.112 | CH3OCH3 7(0,7)-6(1,6) EE |
| 111783.628 | CH3OCH3 7(0,7)-6(1,6) EA+AE |
| 111812.238 | CH3OCH3 18(3,15)-18(2,16) AE+EA |
| 111813.810 | CH3OCH3 18(3,15)-18(2,16) EE |
| 111815.382 | CH3OCH3 18(3,15)-18(2,16) AA |
| 111823.024 | HC5N 42-41 |
| 111827.600 | unidentified |
| 111943.569 | CH3CH2CN 18(2,17)-18(1,18) |
| 111957.756 | CH313CH2CN 13(1,13)-12(1,12) |
| 111967.000 | unidentified |
| 111967.000 | unidentified |
| 112006.000 | unidentified |
| 112016.000 | K37Cl 15-14 |
| 112035.000 | unidentified |
| 112063.440 | MgCN 11-10 J=21/2-19/2 |
| 112078.440 | MgCN 11-10 J=23/2-21/2 |
| 112101.557 | CH3CH213CN 13(1,13)-12(1,12) |
| 112114.000 | unidentified |
| 112150.000 | unidentified |
| 112166.938 | l-C3H 2S 5-4 J=11/2-9/2 1v4 |
| 112248.722 | CH3CHO 6(1,6) - 5(1,5) A++ |
| 112254.512 | CH3CHO 6(-1,6)-5(-1,5) E |
| 112254.512 | CH3CHO 6(-1,6) - 5(-1,5) E |
| 112348.000 | unidentified |
| 112355.480 | 30SiC2 5(0,5)-4(0,4) |
| 112358.780 | C17O 1-0 F=3/2-5/2 |
| 112358.988 | C17O 1-0 F=7/2-5/2 |
| 112360.005 | C17O 1-0 F=5/2-5/2 |
| 112373.548 | (CH3)2CO 11(0,11)-10(1,10) EE |
| 112373.548 | (CH3)2CO 11(1,11)-10(0,10) EE |
| 112381.029 | (CH3)2CO 11(0,11)-10(1,10) AA |
| 112381.029 | (CH3)2CO 11(1,11)-10(0,10) AA |
| 112432.300 | HCOOH 5(4,*)-4(4,*) |
| 112459.610 | HCOOH 5(3,3)-4(3,2) |
| 112467.000 | HCOOH 5(3,2)-4(3,1) |
| 112532.000 | unidentified |
| 112585.000 | unidentified |
| 112593.440 | Si13CC 5(0,5)-4(0,4) |
| 112646.233 | CH3CH2CN 13(1,13)-12(1,12) |
| 112654.117 | NH2CHO 8(3,6)-9(2,7) |
| 112807.096 | t-CH3CH2OH 2(2,1)-1(1,0) |
| 112840.644 | CH2CHCN 12(0,12)-11(0,11) |
| 112869.993 | CH3OCHO 14(3,11)-13(4,10) A |
| 112874.000 | unidentified |
| 112891.430 | HCOOH 5(2,3)-4(2,2) n,t |
| 112893.846 | C6H- 41-40 |
| 112922.500 | C4H 21/2 J=23/2-21/2 1v7e |
| 112997.000 | unidentified |
| 112999.982 | CH3OCH3 20(3,17)-20(2,18) AE+EA |
| 113001.218 | CH3OCH3 20(3,17)-20(2,18) EE |
| 113002.455 | CH3OCH3 20(3,17)-20(2,18) AA |
| 113032.110 | CH2CHCN 8(1,8)-7(0,7) |
| 113059.350 | CH3OCH3 17(3,14)-17(2,15) EE |
| 113061.121 | CH3OCH3 17(3,14)-17(2,15) AA |
| 113090.136 | 13CH3CH2CN 13(2,12)-12(2,11) |
| 113123.337 | CN 1-0 J=1/2-1/2 F=1/2-1/2 |
| 113136.200 | N34S J=5/2-3/2 F=3/2-3/2 e |
| 113144.192 | CN 1-0 J=1/2-1/2 F=1/2-3/2 |
| 113159.000 | unidentified |
| 113170.528 | CN 1-0 J=1/2-1/2 F=3/2-1/2 |
| 113191.317 | CN 1-0 J=1/2-1/2 F=3/2-3/2 |
| 113246.000 | unidentified |
| 113255.669 | CH313CH2CN 13(0,13)-12(0,12) |
| 113260.000 | unidentified |
| 113265.900 | C4H 21/2 J=23/2-21/2 1v7f |
| 113266.690 | CH2CHCN 20(2,18)-20(1,19) |
| 113276.031 | CH3OCH3 10(6,4)-11(5,7) EE |
| 113279.138 | CH3OCH3 10(6,5)-11(5,6) EE |
| 113279.455 | CH3OCH3 10(6,4)-11(5,7) AA |
| 113280.593 | CH3OCH3 10(6,5)-11(5,6) AA |
| 113314.000 | unidentified |
| 113322.000 | unidentified |
| 113326.000 | unidentified |
| 113350.800 | CH3OD 6(1,5)-6(0,6) E |
| 113410.204 | CCS N,J=9,8-8,7 |
| 113432.872 | CH3CH213CN 13(0,13)-12(0,12) |
| 113484.722 | D2CS 4(0,4)-3(0,3) |
| 113488.140 | CN 1-0 J=3/2-1/2 F=3/2-1/2 |
| 113490.982 | CN 1-0 J=3/2-1/2 F=5/2-3/2 |
| 113499.639 | CN 1-0 J=3/2-1/2 F=1/2-1/2 |
| 113508.944 | CN 1-0 J=3/2-1/2 F=3/2-3/2 |
| 113520.414 | CN 1-0 J=3/2-1/2 F=1/2-3/2 |
| 113546.000 | unidentified |
| 113557.000 | unidentified |
| 113566.000 | unidentified |
| 113617.453 | 13CH3CH2CN 13(7,*)-12(7,*) |
| 113621.133 | 13CH3CH2CN 13(6,*)-12(6,*) |
| 113623.000 | unidentified |
| 113623.392 | 13CH3CH2CN 13(8,*)-12(8,*) |
Spectral window: 2
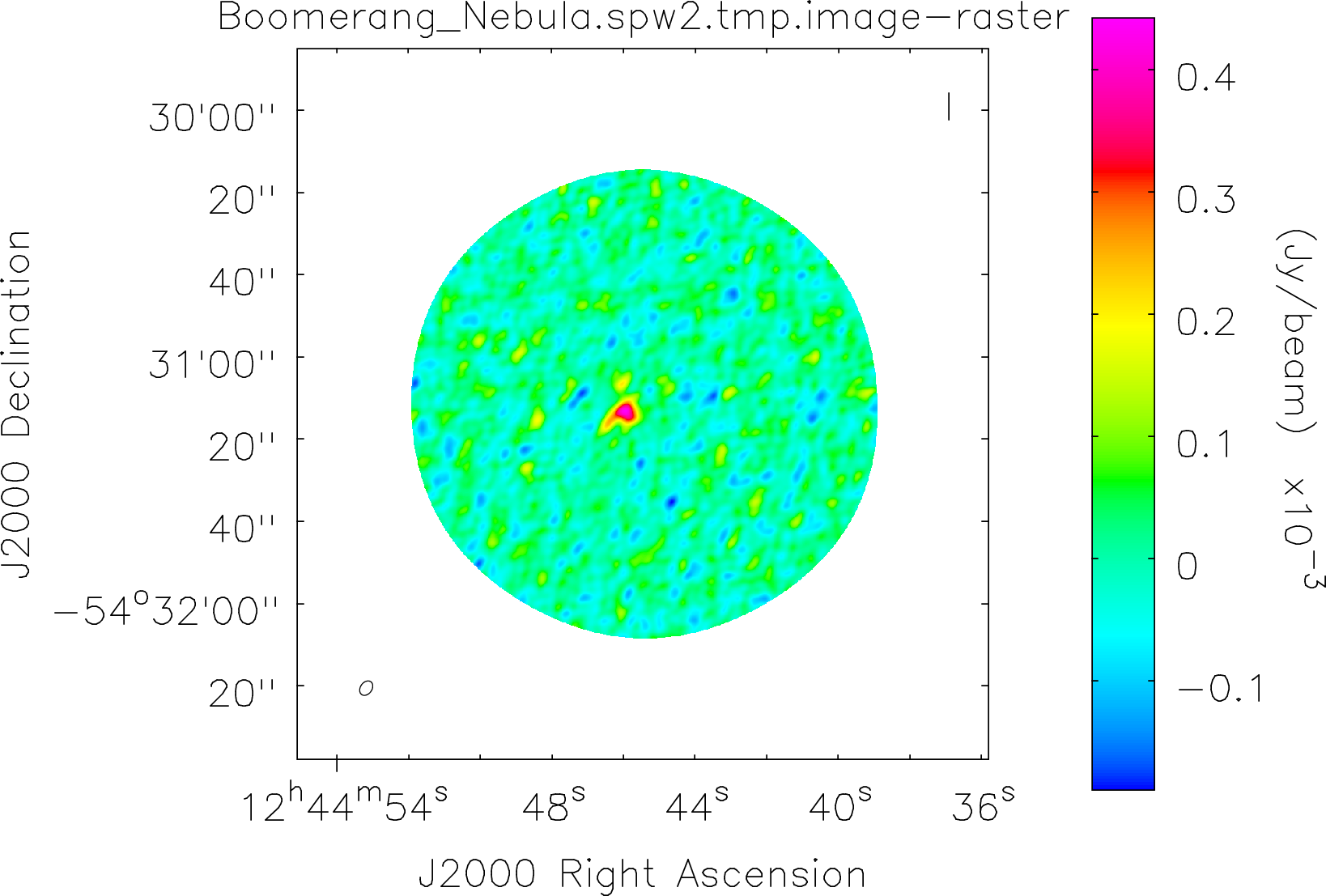
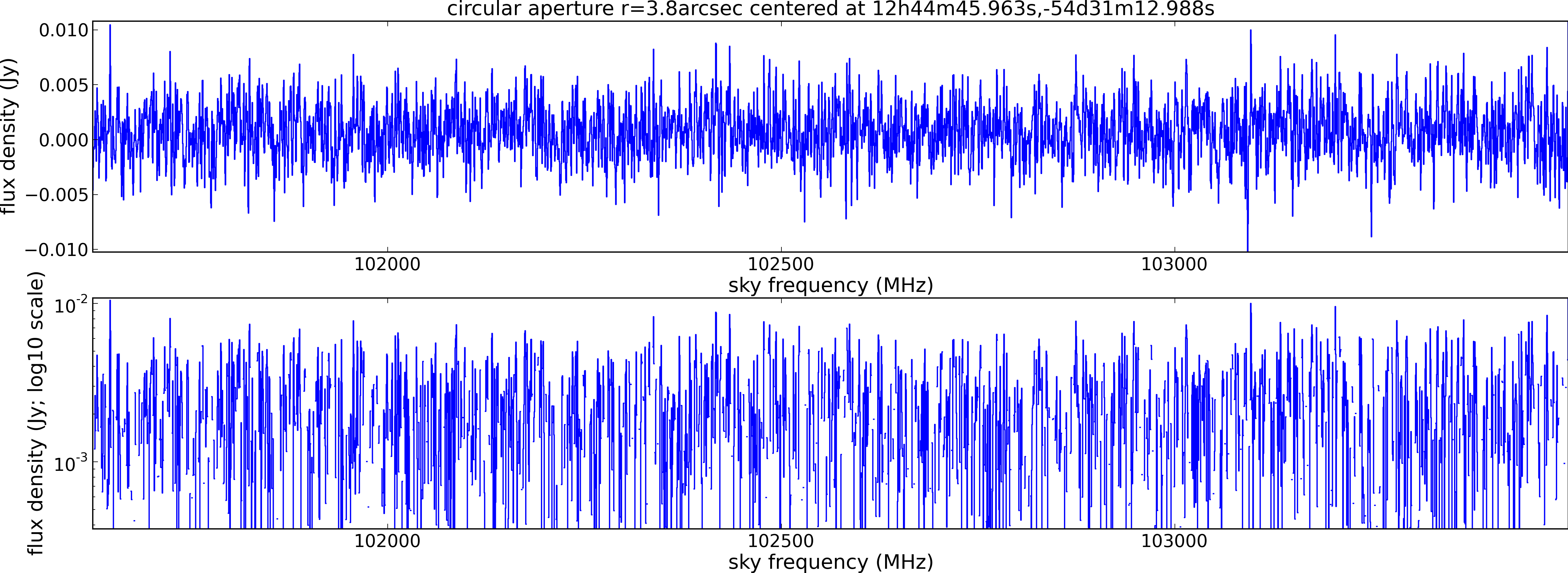
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 101626.822 | CH3OCHO 9(1,9)-8(0,8) E |
| 101628.167 | CH3OCHO 9(1,9)-8(0,8) A |
| 101637.231 | CH2CHCN 11(1,11)-10(1,10) |
| 101659.000 | unidentified |
| 101668.700 | unidentified |
| 101677.000 | unidentified |
| 101688.880 | 33SO2 12(4,8)-12(3,11) |
| 101690.002 | CH3CH2CN 27(2,25)-27(1,26) |
| 101708.800 | unidentified |
| 101713.600 | unidentified |
| 101737.211 | CH3OH 9(-2,8)-9(1,8) E |
| 101771.892 | CH3OCHO 24(5,19)-24(4,20) A |
| 101873.641 | C6H 21/2 J=73/2-71/2 f |
| 101881.306 | C6H- 37-36 |
| 101892.560 | MgCN 10-9 J=21/2-19/2 |
| 101925.513 | C6H 21/2 J=73/2-71/2 e |
| 101961.512 | Na37Cl 8-7 |
| 101970.000 | unidentified |
| 101981.426 | CH2CO 5(1,4)-4(1,3) |
| 102031.874 | 34SO2 3(1,3)-2(0,2) |
| 102031.940 | Al35Cl 7-6 |
| 102043.000 | unidentified |
| 102064.263 | NH2CHO 5(1,5)-4(1,4) |
| 102065.856 | H2COH+ 4(0,4)-3(1,3) |
| 102122.701 | CH3OH 10(-2,9)-10(1,9) E |
| 102178.829 | 13CH2CHCN 11(2,9)-10(2,8) |
| 102202.490 | CH3SH 4( 1)-3( 1) A-- |
| 102217.571 | NH2CHO 2(1,2)-1(0,1) |
| 102274.000 | unidentified |
| 102298.085 | HCCCHO 11(0,11)-10(0,10) |
| 102319.000 | unidentified |
| 102375.000 | unidentified |
| 102399.000 | unidentified |
| 102405.662 | C4H- 11-10 |
| 102407.000 | unidentified |
| 102423.000 | unidentified |
| 102432.000 | unidentified |
| 102489.386 | g-CH3CH2OH 11(1,11)-11(0,11) t=1-0 |
| 102516.635 | CH3CCH 6(4)-5(4) |
| 102530.346 | CH3CCH 6(3)-5(3) |
| 102533.756 | (CH3)2CO 10(*,10)-9(*,9) EE t=1 |
| 102540.143 | CH3CCH 6(2)-5(2) |
| 102546.023 | CH3CCH 6(1)-5(1) |
| 102547.983 | CH3CCH 6(0)-5(0) |
| 102554.696 | (CH3)2CO 10(0,10)-9(1,9) EE |
| 102562.281 | (CH3)2CO 10(0,10)-9(1,9) AA |
| 102587.476 | CF+ 1-0 |
| 102636.230 | C5H 21/2 J=43/2-41/2 e |
| 102640.000 | unidentified |
| 102642.660 | C5H 21/2 J=43/2-41/2 f |
| 102644.000 | unidentified |
| 102650.000 | unidentified |
| 102658.096 | CH3OH 11(-2,10)-11(1,10) E |
| 102690.055 | SO2 33(8,26)-34(7,27) |
| 102734.338 | CH3OCHO 16(5,11)-16(4,12) E |
| 102736.773 | CH3OCHO 16(5,11)-16(4,12) A |
| 102807.370 | H2C34S 3(1,2)-2(1,1) |
| 102916.085 | SiC3 9(0,9)-8(0,8) |
| 102952.042 | 13CH3CH2CN 12(0,12)-11(0,11) |
| 102957.990 | CH3OH 15(-2,13)-16(-3,13) E t=1 |
| 102992.345 | H2CCC 5(1,5)-4(1,4) |
| 103028.000 | unidentified |
| 103040.548 | H2CS 3(0,3)-2(0,2) |
| 103051.867 | H2CS 3(2,1)-2(2,0) |
| 103071.000 | unidentified |
| 103075.000 | unidentified |
| 103114.824 | CH3OCHO 21(4,17)-21(3,18) A |
| 103187.375 | NH2D 8(3,6)-8(2,6) F=8-9 U |
| 103196.000 | unidentified |
| 103216.600 | unidentified |
| 103227.000 | unidentified |
| 103266.000 | C4H 21/2 J=21/2-19/2 1v7e |
| 103297.000 | unidentified |
| 103319.287 | l-C3H 23/2 J=9/2-7/2 F=5-4f |
| 103319.818 | l-C3H 23/2 J=9/2-7/2 F=4-3f |
| 103328.000 | unidentified |
| 103329.920 | C5H 23/2 J=45/2-43/2 f |
| 103372.506 | l-C3H 23/2 J=9/2-7/2 F=5-4e |
| 103373.129 | l-C3H 23/2 J=9/2-7/2 F=4-3e |
| 103376.784 | CH3OCHO 24(6,18)-24(5,17) A |
| 103381.209 | CH3OH 12(-2,11)-12(1,11) E |
| 103387.227 | CH3OCHO 24(6,18)-24(5,17) E |
| 103391.283 | CH2OHCHO 10(0,10)-9(1,9) |
| 103402.000 | unidentified |
| 103419.673 | CH313CH2CN 12(1,12)-11(1,11) |
| 103466.479 | CH3OCHO 8(2,6)-7(2,5) E |
| 103478.699 | CH3OCHO 8(2,6)-7(2,5) A |
Spectral window: 3
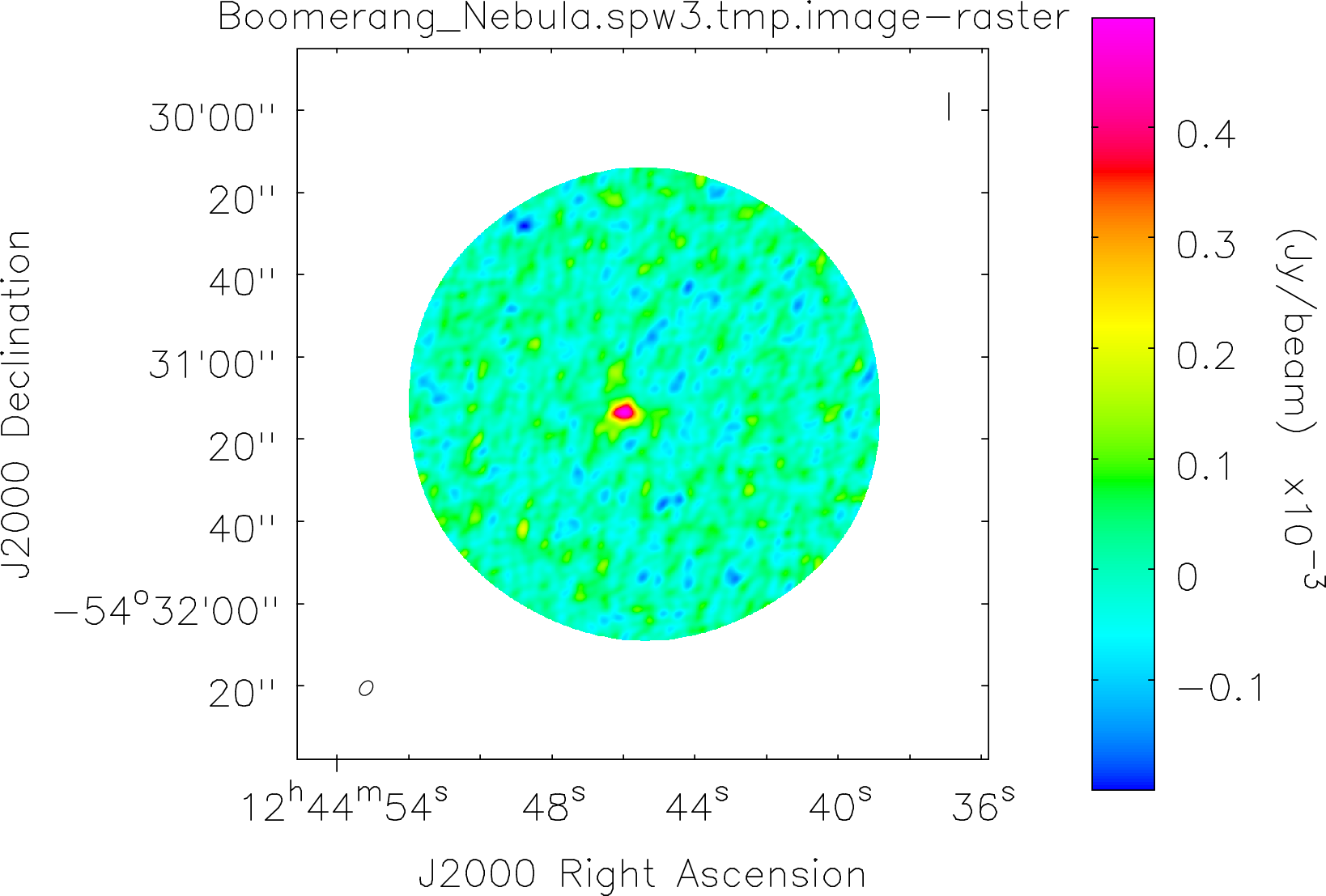
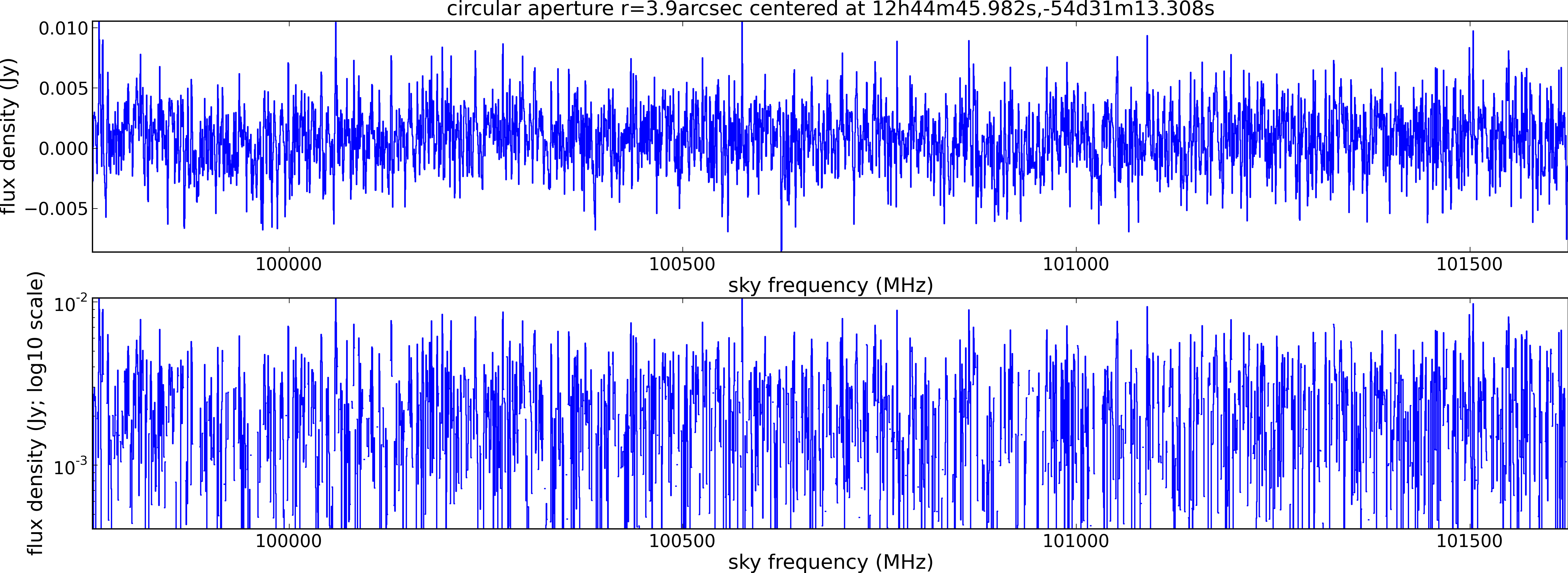
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 99774.130 | H2C34S 3(1,3)-2(1,2) |
| 99866.509 | CCS N,J=8,7-7,6 |
| 99903.000 | unidentified |
| 99929.540 | K35Cl 13-12 |
| 99953.270 | NH2CN 5(2,4)-4(2,3) |
| 99956.600 | NH2CN 5(2,3)-4(2,2) |
| 99972.660 | NH2CN 5(0,5)-4(0,4) |
| 100029.565 | SO N,J=5,4-4,4 |
| 100076.386 | HCCCN 11-10 |
| 100094.500 | CH2CO 5(1,5)-4(1,4) |
| 100109.744 | CH3CH213CN 11(1,10)-10(1,9) |
| 100110.270 | CH3SH 4(1)-3(1) A++ |
| 100122.000 | unidentified |
| 100155.839 | CH313CH2CN 11(1,10)-10(1,9) |
| 100157.000 | unidentified |
| 100173.100 | CH3SH 7(2)-8(1) A++ |
| 100185.000 | unidentified |
| 100197.200 | unidentified |
| 100200.400 | unidentified |
| 100240.584 | HCCCN 11-10 1v6 =1e |
| 100294.508 | CH3OCHO 8(3,5)-7(3,4) E |
| 100308.210 | CH3OCHO 8(3,5)-7(3,4) A |
| 100322.411 | HCCCN 11-10 1v7 =1e |
| 100332.000 | unidentified |
| 100365.000 | unidentified |
| 100373.000 | unidentified |
| 100421.000 | unidentified |
| 100436.000 | unidentified |
| 100452.072 | g-CH3CH2OH 24(2,23)-24(1,23) t=1-0 |
| 100460.412 | CH3OCH3 6(2,5)-6(1,6) EA+AE |
| 100463.066 | CH3OCH3 6(2,5)-6(1,6) EE |
| 100465.708 | CH3OCH3 6(2,5)-6(1,6) AA |
| 100466.175 | HCCCN 11-10 1v7 =1f |
| 100482.174 | CH3OCHO 8(1,7)-7(1,6) E |
| 100490.715 | CH3OCHO 8(1,7)-7(1,6) A |
| 100491.721 | N2O 4-3 |
| 100498.500 | unidentified |
| 100509.000 | unidentified |
| 100526.506 | CH3NC 5-4 |
| 100543.246 | CH2CN 5(2,3)-4(2,2) J=9/2-7/2 |
| 100598.067 | CH2CN 5(0,5)-4(0,4) J=11/2-9/2 |
| 100611.012 | CH2CN 5(0,5)-4(0,4) J=9/2-7/2 |
| 100614.291 | CH3CH2CN 11(1,10)-10(1,9) |
| 100629.500 | NH2CN 5(1,4)-4(1,3) |
| 100631.499 | CH2CN 5(2,4)-4(2,3) J=11/2-9/2 |
| 100638.870 | CH3OH 13(2,11)-12(3,9) E |
| 100681.476 | CH3OCHO 9(0,9)-8(0,8) E |
| 100683.392 | CH3OCHO 9(0,9)-8(0,8) A |
| 100708.784 | HCCCN 11-10 2v7 =0 |
| 100711.064 | HCCCN 11-10 2v7 =2 e |
| 100714.395 | HCCCN 11-10 2v7 =2 f |
| 100841.300 | unidentified |
| 100855.437 | CH3COOH 9(-1,9)-8(-1,8) E |
| 100855.437 | CH3COOH 9(-1,9)-8(0,8) E |
| 100855.437 | CH3COOH 9(0,9)-8(-1,8) E |
| 100855.437 | CH3COOH 9(0,9)-8(0,8) E |
| 100856.600 | unidentified |
| 100864.800 | unidentified |
| 100866.300 | unidentified |
| 100878.105 | SO2 2(2,0)-3(1,3) |
| 100897.459 | CH3COOH 9(*,9)-8(*,8) A |
| 100898.580 | CH3SH 7(1)-7(0) E |
| 100911.136 | CH2CHCH3 6(1,6)-5(1,5) E |
| 100911.401 | CH2CHCH3 6(1,6)-5(1,5) A |
| 100990.034 | t-CH3CH2OH 8(2,7)-8(1,8) |
| 101002.355 | CH2CO 5(3,3)-4(3,2) |
| 101002.360 | CH2CO 5(3,2)-4(3,1) |
| 101024.438 | CH2CO 5(2,4)-4(2,3) |
| 101029.750 | CH3SH 4(-1)-3(-1) E |
| 101036.589 | CH2CO 5(0,5)-4(0,4) |
| 101139.160 | CH3SH 4(0)-3(0) A |
| 101139.650 | CH3SH 4(0)-3(0) E |
| 101159.460 | CH3SH 4(2)-3(2) A-- |
| 101167.150 | CH3SH 4(-2)-3(-2) E |
| 101168.340 | CH3SH 4(2)-3(2) E |
| 101174.677 | HC5N 38-37 |
| 101179.760 | CH3SH 4(2)-3(2) A |
| 101180.347 | C6H 23/2 J=73/2-71/2 e |
| 101185.367 | CH3OH 6(-2,5)-6(1,5) E |
| 101200.400 | unidentified |
| 101211.500 | unidentified |
| 101215.324 | C6H 23/2 J=73/2-71/2 f |
| 101243.600 | unidentified |
| 101253.800 | unidentified |
| 101272.900 | unidentified |
| 101278.999 | CH3OCHO 9(1,9)-8(0,8) E t=1 |
| 101279.200 | unidentified |
| 101284.360 | CH3SH 4( 1)-3( 1) E |
| 101284.400 | H2C34S 3(0,3)-2(0,2) |
| 101287.300 | unidentified |
| 101293.328 | CH3OH 7(-2,6)-7(1,6) E |
| 101299.309 | NH2CHO 18(2,16)-18(2,17) |
| 101302.116 | CH3OCHO 25(6,19)-25(5,20) A |
| 101305.534 | CH3OCHO 25(6,19)-25(5,20) E |
| 101314.830 | DCCCN 12-11 |
| 101318.600 | unidentified |
| 101332.993 | H2CO 6(1,5)-6(1,6) |
| 101343.448 | CH3CHO 3(-1,3) - 2(0,2) E |
| 101348.800 | unidentified |
| 101357.000 | unidentified |
| 101371.000 | unidentified |
| 101382.335 | DNCO 5(1,5)-4(1,4) |
| 101408.900 | unidentified |
| 101414.723 | CH3OCHO 13(3,11)-13(2,12) A |
| 101426.664 | (CH3)2CO 9(1,8)-8(2,7) AE |
| 101426.716 | (CH3)2CO 9(1,8)-8(2,7) AE,EA |
| 101426.759 | (CH3)2CO 9(1,8)-8(2,7) EA |
| 101427.041 | (CH3)2CO 9(2,8)-8(1,7) AE |
| 101427.090 | (CH3)2CO 9(2,8)-8(1,7) AE,EA |
| 101427.130 | (CH3)2CO 9(2,8)-8(1,7) EA |
| 101435.900 | unidentified |
| 101451.059 | (CH3)2CO 9(1,8)-8(2,7) EE |
| 101451.446 | (CH3)2CO 9(2,8)-8(1,7) EE |
| 101469.719 | CH3OH 8(-2,7)-8(1,7) E |
| 101477.885 | H2CS 3(1,3)-2(1,2) |
| 101499.200 | unidentified |
| 101503.800 | unidentified |
| 101523.600 | unidentified |
| 101531.974 | CH2CN 5(1,4)-4(1,3) J=11/2-9/2 |
| 101534.000 | H13COOH 9(3,6)-10(2,9) |
| 101545.423 | CH3OCHO 18(3,15)-18(3,16) A |
| 101553.275 | 13CH3CH2CN 12(1,12)-11(1,11) |
| 101559.383 | CH3OCH3 12(2,10)-11(3,9) AA |
| 101560.264 | 33SO2 17(5,13)-18(4,14) |
| 101562.117 | CH3OCH3 12(2,10)-11(3,9) EE |
| 101564.832 | CH3OCH3 12(2,10)-11(3,9) AE |
| 101564.872 | CH3OCH3 12(2,10)-11(3,9) EA |
| 101575.500 | unidentified |
uv sampling
| FieldID | SpwID | uv_min(klambda) | uv_max(klambda) |
|---|---|---|---|
| 0 | 0 | 13.122 | 265.315 |
| 0 | 1 | 13.115 | 265.358 |
| 0 | 2 | 13.109 | 265.416 |
| 0 | 3 | 13.108 | 265.424 |
| 2 | 0 | 15.655 | 200.949 |
| 2 | 1 | 15.655 | 200.949 |
| 2 | 2 | 15.655 | 200.949 |
| 2 | 3 | 15.655 | 200.949 |
| 4 | 0 | 14.459 | 264.230 |
| 4 | 1 | 14.458 | 264.211 |
| 4 | 2 | 14.458 | 264.191 |
| 4 | 3 | 14.458 | 264.188 |
| 5 | 0 | 14.423 | 264.228 |
| 5 | 1 | 14.423 | 264.208 |
| 5 | 2 | 14.423 | 264.187 |
| 5 | 3 | 14.423 | 264.184 |
| 6 | 0 | 14.431 | 264.258 |
| 6 | 1 | 14.430 | 264.238 |
| 6 | 2 | 14.430 | 264.217 |
| 6 | 3 | 14.430 | 264.214 |
| 7 | 0 | 14.442 | 264.287 |
| 7 | 1 | 14.441 | 264.268 |
| 7 | 2 | 14.441 | 264.247 |
| 7 | 3 | 14.441 | 264.244 |
| 8 | 0 | 14.452 | 264.316 |
| 8 | 1 | 14.452 | 264.297 |
| 8 | 2 | 14.451 | 264.276 |
| 8 | 3 | 14.451 | 264.273 |
| 9 | 0 | 14.471 | 264.332 |
| 9 | 1 | 14.470 | 264.313 |
| 9 | 2 | 14.470 | 264.294 |
| 9 | 3 | 14.470 | 264.291 |
| 10 | 0 | 14.494 | 264.411 |
| 10 | 1 | 14.493 | 264.394 |
| 10 | 2 | 14.493 | 264.375 |
| 10 | 3 | 14.493 | 264.372 |
| 11 | 0 | 15.913 | 261.940 |
| 11 | 1 | 15.913 | 261.940 |
| 11 | 2 | 15.913 | 261.940 |
| 11 | 3 | 15.913 | 261.940 |
| 12 | 0 | 16.485 | 270.153 |
| 12 | 1 | 16.485 | 270.153 |
| 12 | 2 | 16.485 | 270.153 |
| 12 | 3 | 16.485 | 270.153 |