2011.0.00471.S/2011.0.00471.S_2012-05-29/2011.0.00471.S_2012-05-29/sg_ouss_id/group_ouss_id/member_ouss_id/calibrated
full summary of this dataset can be found here
Spectral setup
| SpwID | Frame | #chan | skyfreq(MHz) | Chwid(kHz) | BW(MHz) | beam("x" deg) |
|---|---|---|---|---|---|---|
| 0 | TOPO | 3840 | 97422.8 ~ 98360.1 | 244.141 | 937.500000 | 2.4 x 1.6, 68.6 |
| 1 | TOPO | 3840 | 98463.9 ~ 99401.1 | 244.141 | 937.500000 | 2.4 x 1.6, 67.3 |
| 2 | TOPO | 3840 | 85937.4 ~ 86874.6 | 244.141 | 937.500000 | 2.8 x 1.8, 69.3 |
| 3 | TOPO | 3840 | 88354.1 ~ 89291.3 | 244.141 | 937.500000 | 2.7 x 1.8, 70.5 |
Observed fields
| FieldID | FieldName | R.A. | Dec | Epoch | nRows |
|---|---|---|---|---|---|
| 0 | J0538-440 | 05:38:50.361000 | -44.05.08.93900 | J2000 | 55080 |
| 1 | J0637-752 | 06:35:46.507000 | -75.16.16.81500 | J2000 | 45900 |
| 3 | 30_Doradus | 05:38:44.488191 | -69.04.12.94344 | J2000 | 73440 |
| 4 | 30_Doradus | 05:38:49.407087 | -69.04.12.92004 | J2000 | 73440 |
| 5 | 30_Doradus | 05:38:42.032531 | -69.04.35.74914 | J2000 | 73440 |
| 6 | 30_Doradus | 05:38:46.951427 | -69.04.35.72573 | J2000 | 73440 |
| 7 | 30_Doradus | 05:38:51.870323 | -69.04.35.70232 | J2000 | 73440 |
| 8 | 30_Doradus | 05:38:44.495768 | -69.04.58.53142 | J2000 | 73440 |
| 9 | 30_Doradus | 05:38:49.414664 | -69.04.58.50801 | J2000 | 73440 |
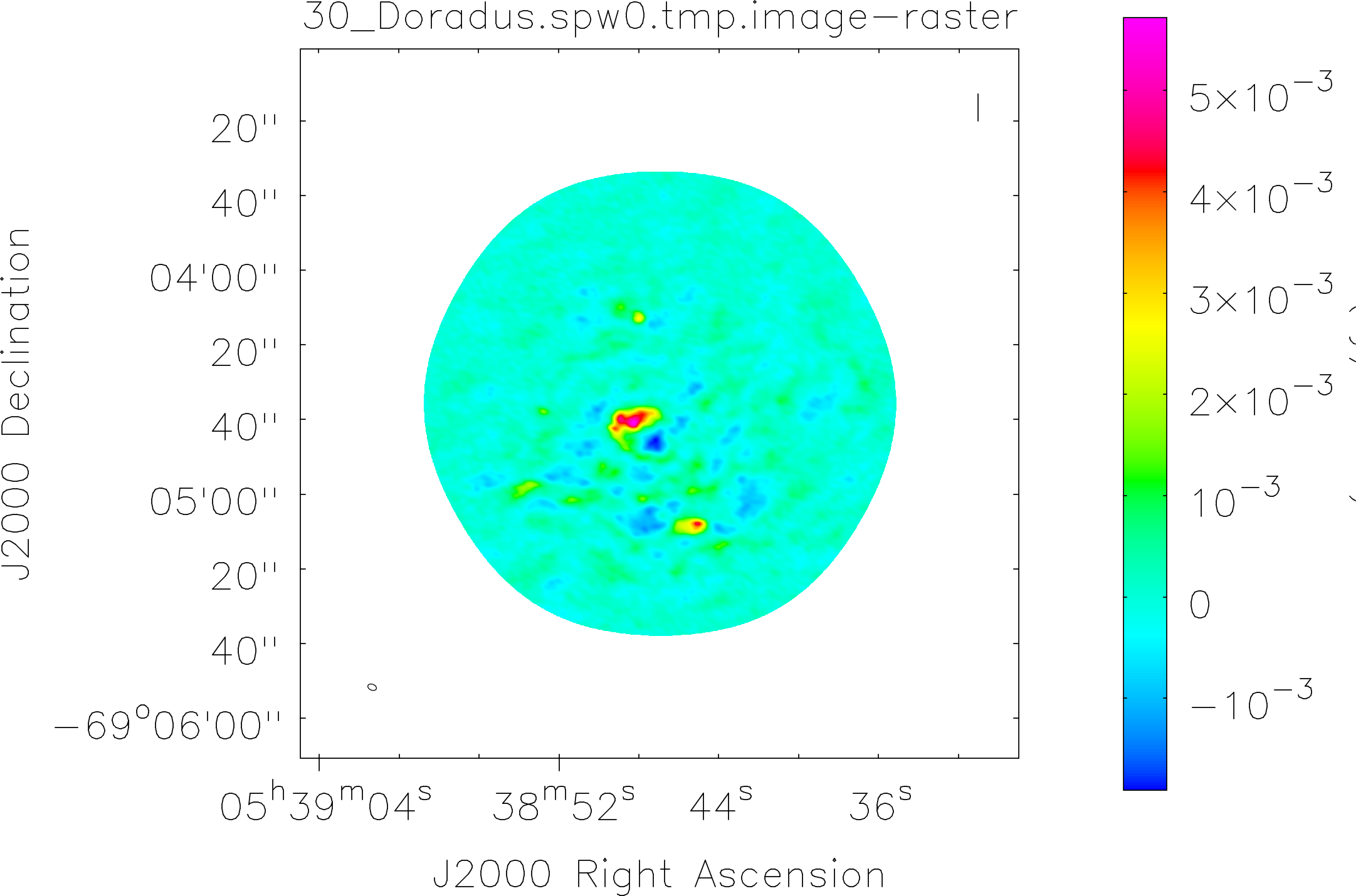
Spectral window: 0
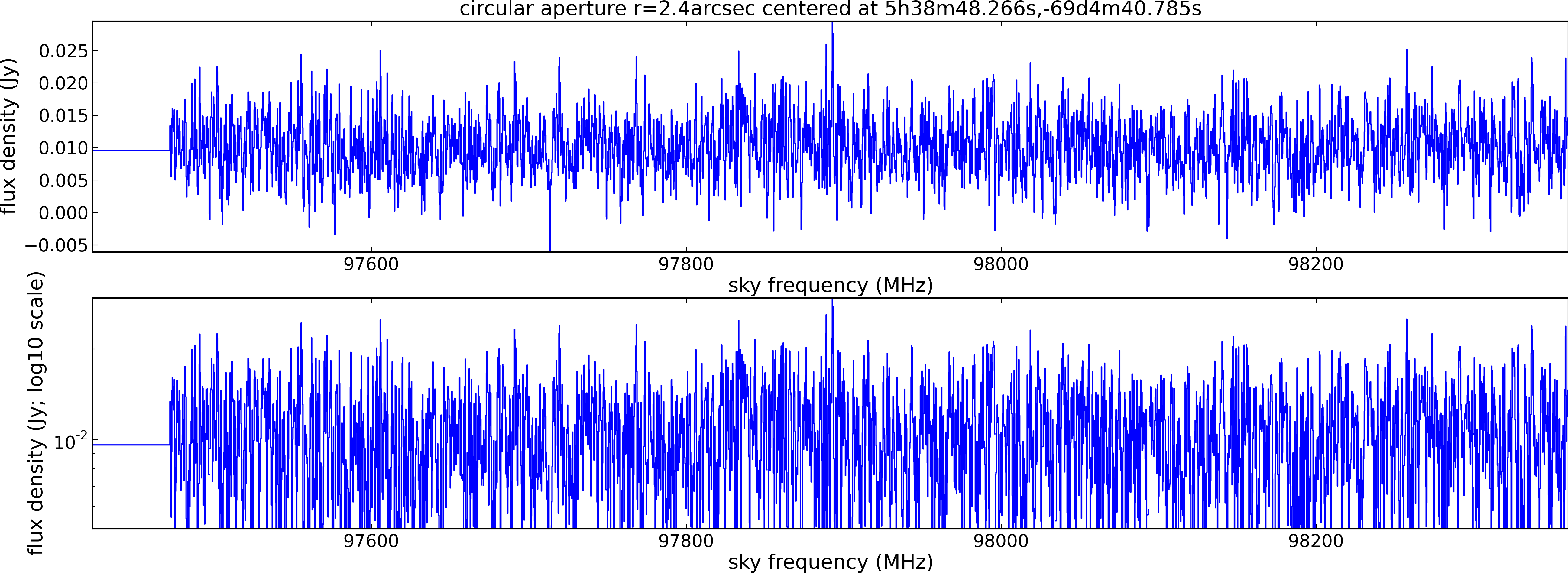
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 97535.908 | g-CH3CH2OH 21(1,21)-21(0,21) t=1-0 |
| 97536.849 | g-CH3CH2OH 23(1,23)-23(0,23) t=1-0 |
| 97546.875 | g-CH3CH2OH 29(1,28)-29(2,28) t=1-0 |
| 97549.692 | g-CH3CH2OH 26(0,26)-26(1,26) t=1-0 |
| 97562.844 | g-CH3CH2OH 24(1,24)-24(0,24) t=1-0 |
| 97569.000 | unidentified |
| 97574.042 | g-CH3CH2OH 20(1,20)-20(0,20) t=1-0 |
| 97577.278 | CH3OCHO 8(5,3)-7(5,2) E t=1 |
| 97577.900 | unidentified |
| 97582.808 | CH3OH 2(1,1)-1(1,0) A-- |
| 97597.180 | CH3OCHO 8(3,6)-7(3,5) A t=1 |
| 97600.390 | g-CH3CH2OH 25(1,25)-25(0,25) t=1-0 |
| 97603.000 | unidentified |
| 97618.700 | unidentified |
| 97631.329 | g-CH3CH2OH 27(0,27)-27(1,27) t=1-0 |
| 97632.700 | H213CS 3(1,3)-2(1,2) |
| 97649.502 | g-CH3CH2OH 19(1,19)-19(0,19) t=1-0 |
| 97651.392 | CH3OCHO 10(4,7)-10(3,8) E |
| 97661.356 | CH3OCHO 8(4,5)-7(4,4) A t=1 |
| 97662.000 | unidentified |
| 97672.030 | CH313CH2CN 11(2,10)-10(2,9) |
| 97678.260 | CH3OH 21(6,16)-22(5,17) A-- |
| 97679.380 | CH3OH 21(6,15)-22(5,18) A++ |
| 97694.197 | CH3OCHO 10(4,7)-10(3,8) A |
| 97702.340 | SO2 7(3,5)-8(2,6) |
| 97715.401 | 34SO N,J=2,3-1,2 |
| 97729.400 | unidentified |
| 97738.762 | CH3OCHO 8(6,3)-7(6,2) E t=1 |
| 97739.300 | unidentified |
| 97752.824 | CH3OCHO 8(4,4)-7(4,3) A t=1 |
| 97753.400 | unidentified |
| 97755.610 | g-CH3CH2OH 28(1,28)-28(0,28) t=1-0 |
| 97774.307 | g-CH3CH2OH 18(1,18)-18(0,18) t=1-0 |
| 97815.987 | g-CH3CH2OH 29(1,29)-29(0,20) t=1-0 |
| 97833.634 | H2CCCC 11(1,11)-10(1,10) |
| 97846.300 | unidentified |
| 97862.540 | C5H 21/2 J=41/2-39/2 e |
| 97868.730 | C5H 21/2 J=41/2-39/2 f |
| 97869.800 | unidentified |
| 97874.000 | unidentified |
| 97885.626 | CH3OCHO 8(5,4)-7(5,3) E t=1 |
| 97886.000 | unidentified |
| 97897.129 | CH3OCHO 8(4,4)-7(4,3) E t=1 |
| 97897.500 | unidentified |
| 97915.600 | unidentified |
| 97926.000 | unidentified |
| 97931.200 | unidentified |
| 97932.445 | g-CH3CH2OH 31(0,31)-31(1,31) t=1-0 |
| 97957.200 | unidentified |
| 97962.858 | g-CH3CH2OH 17(1,17)-17(0,17) t=1-0 |
| 97980.953 | CS 2-1 |
| 97995.212 | l-C3H 21/2 J=9/2-7/2 F=5-4e |
| 97995.951 | l-C3H 21/2 J=9/2-7/2 F=4-3e |
| 98011.649 | l-C3H 21/2 J=9/2-7/2 F=5-4f |
| 98012.576 | l-C3H 21/2 J=9/2-7/2 F=4-3f |
| 98030.400 | CH3OH 24(6,19)-23(7,16) A-- |
| 98030.440 | CH3OH 24(6,18)-23(7,17) A++ |
| 98033.907 | CH3CH213CN 11(7,*)-10(7,*) |
| 98039.612 | CH313CH2CN 11(7,*)-10(7,*) |
| 98040.565 | CH313CH2CN 11(6,*)-10(6,*) |
| 98042.563 | CH3CH213CN 11(5,*)-10(5,*) |
| 98052.969 | CH313CH2CN 11(5,*)-10(5,*) |
| 98056.191 | CH313CH2CN 11(9,*)-10(9,*) |
| 98060.000 | unidentified |
| 98072.727 | CH3CH213CN 11(4,8)-10(4,7) |
| 98074.628 | CH3CH213CN 11(4,7)-10(4,6) |
| 98087.345 | CH313CH2CN 11(4,8)-10(4,7) |
| 98089.684 | CH313CH2CN 11(4,7)-10(4,6) |
| 98117.426 | CH3CH213CN 11(3,9)-10(3,8) |
| 98134.865 | CH313CH2CN 11(3,9)-10(3,8) |
| 98153.787 | SiC4 32-31 |
| 98165.346 | 13CH3CH2CN 11(1,10)-10(1,9) |
| 98177.578 | CH3CH2CN 11(2,10)-10(2,9) |
| 98182.199 | CH3OCHO 8(7,1)-7(7,0) E |
| 98190.653 | CH3OCHO 8(7,1)-7(7,0) A |
| 98190.653 | CH3OCHO 8(7,2)-7(7,1) A |
| 98191.414 | CH3OCHO 8(7,2)-7(7,1) E |
| 98218.353 | H2CCCC 11(3,9)-10(3,8) |
| 98218.355 | H2CCCC 11(3,8)-10(3,7) |
| 98230.313 | g-CH3CH2OH 16(1,16)-16(0,16) t=1-0 |
| 98238.285 | H2CCCC 11(2,9)-10(2,8) |
| 98244.941 | H2CCCC 11(0,11)-10(0,10) |
| 98257.700 | unidentified |
| 98265.900 | unidentified |
| 98268.515 | CCCS 17-16 |
| 98270.369 | CH3OCHO 8(6,2)-7(6,1) E |
| 98278.870 | CH3OCHO 8(6,3)-7(6,2) E |
| 98279.746 | CH3OCHO 8(6,3)-7(6,2) A |
| 98279.788 | CH3OCHO 8(6,2)-7(6,1) A |
| 98333.900 | unidentified |
| 98351.900 | unidentified |
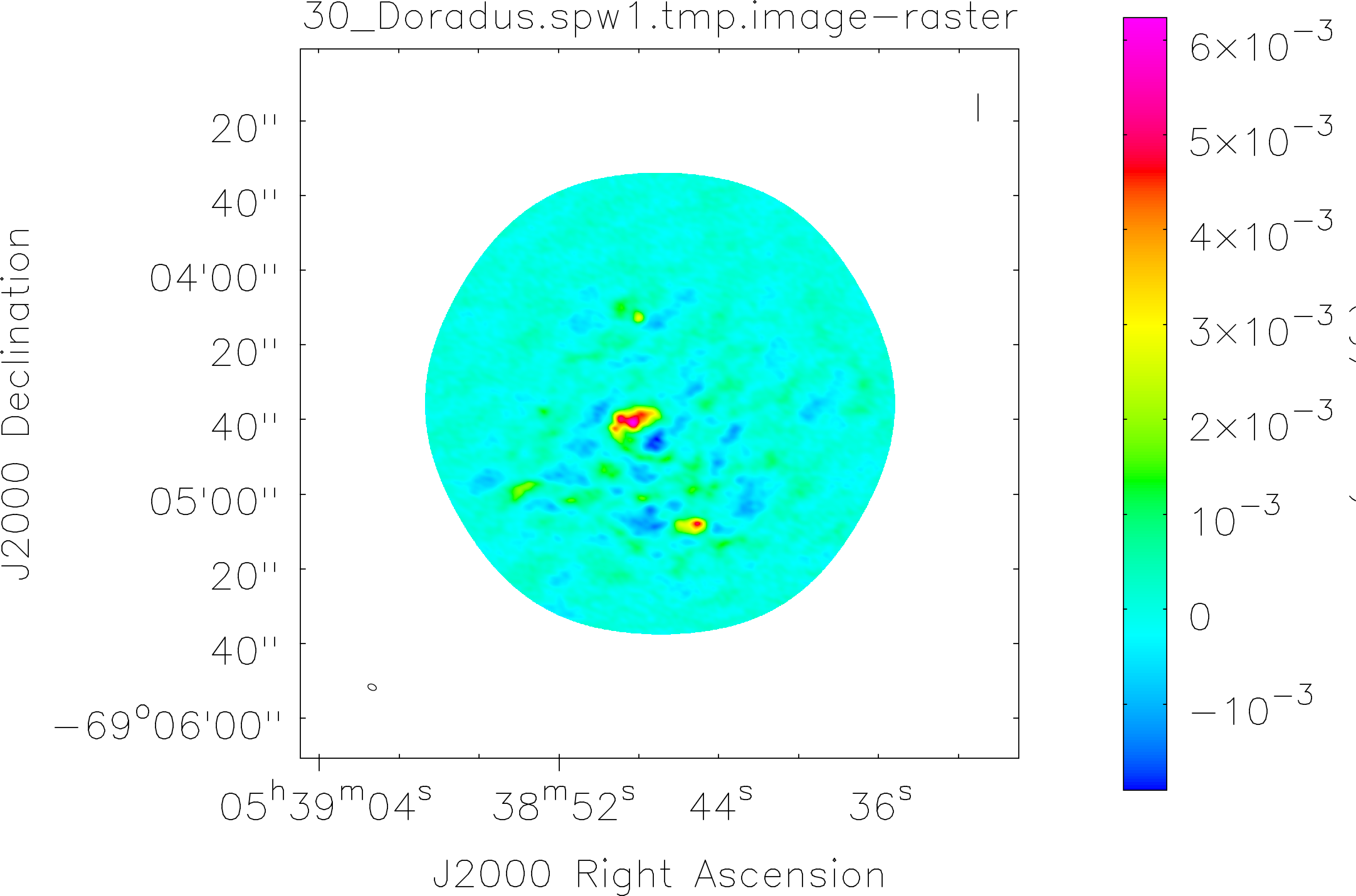
Spectral window: 1
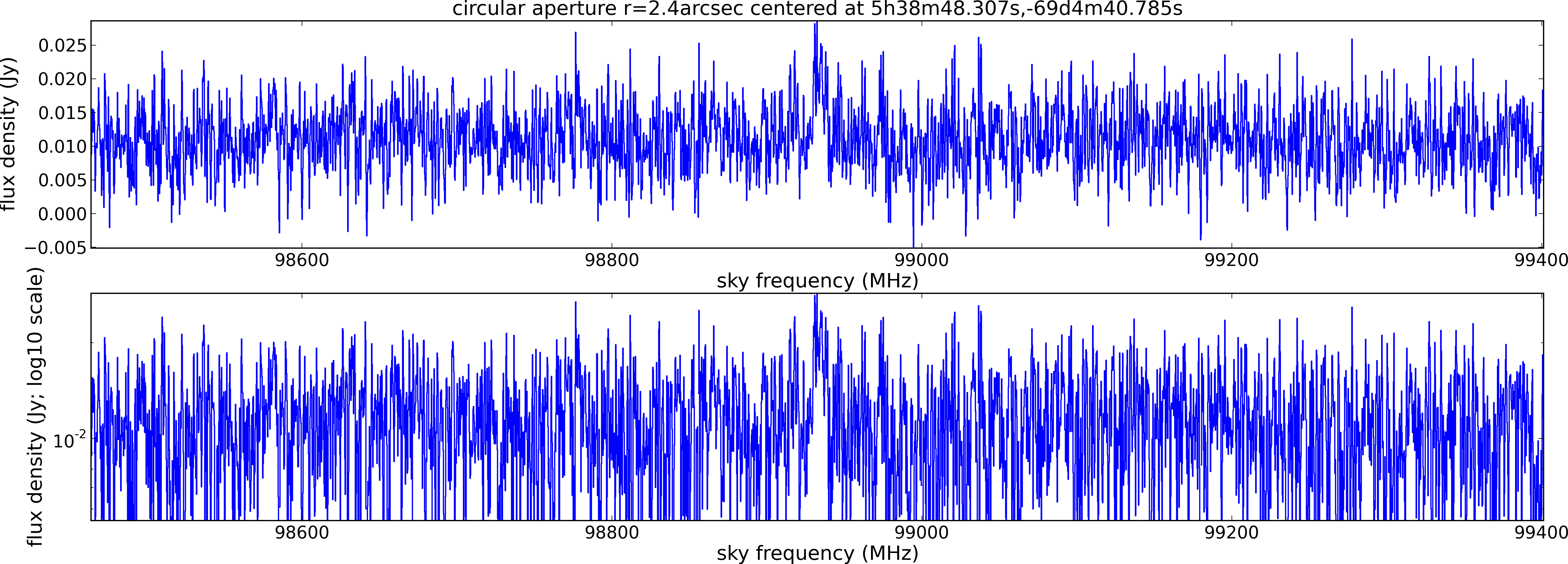
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 98474.550 | 33SO N,J=2,3-1,2 F=3/2-1/2 |
| 98482.150 | 33SO N,J=2,3-1,2 F=5/2-3/2 |
| 98489.080 | 33SO N,J=2,3-1,2 F=7/2-5/2 |
| 98493.680 | 33SO N,J=2,3-1,2 F=9/2-7/2 |
| 98512.519 | HC5N 37-36 |
| 98523.881 | CH3CH2CN 11(6)-10(6) |
| 98524.663 | CH3CH2CN 11(7)-10(7) |
| 98524.690 | C5H 23/2 J=41/2-39/2 f |
| 98527.190 | C5H 23/2 J=41/2-39/2 e |
| 98532.075 | CH3CH2CN 11(8)-10(8) |
| 98533.983 | CH3CH2CN 11(5)-10(5) |
| 98544.152 | CH3CH2CN 11(9,*)-10(9,*) |
| 98564.832 | CH3CH2CN 11(4,8)-10(4,7) |
| 98566.797 | CH3CH2CN 11(4,7)-10(4,6) |
| 98606.771 | CH3OCHO 8(3,6)-7(3,5) E |
| 98610.104 | CH3CH2CN 11(3,9)-10(3,8) |
| 98611.195 | CH3OCHO 8(3,6)-7(3,5) A |
| 98630.000 | unidentified |
| 98651.514 | (CH3)2CO 5(5,1)-4(4,1) EE |
| 98655.097 | H2CCCC 11(1,10)-10(1,9) |
| 98663.000 | unidentified |
| 98682.635 | CH3OCHO 8(4,5)-7(4,4) A |
| 98696.000 | unidentified |
| 98701.106 | CH3CH2CN 11(3,8)-10(3,7) |
| 98711.931 | CH3OCHO 8(4,5)-7(4,4) E |
| 98747.797 | CH3OCHO 8(4,4)-7(4,3) E |
| 98771.000 | unidentified |
| 98792.314 | CH3OCHO 8(4,4)-7(4,3) A |
| 98800.980 | (CH3)2CO 5(5,0)-4(4,0) EE |
| 98863.314 | CH3CHO 5(1,4) - 4(1,3) E |
| 98875.160 | CH3OCHO 11(4,8)-11(3,9) A |
| 98900.951 | CH3CHO 5(1,4) - 4(1,3) A-- |
| 98926.723 | AlF 3-2 |
| 98940.020 | CCCN 10-9 J=21/2-19/2 |
| 98958.780 | CCCN 10-9 J=19/2-17/2 |
| 98976.278 | SO2 28(7,21)-29(6,24) |
| 99011.000 | unidentified |
| 99068.000 | unidentified |
| 99083.405 | C6H 21/2 J=71/2-69/2 f |
| 99087.000 | unidentified |
| 99118.600 | NH2D 5(2,4)-4(1,4) |
| 99120.000 | unidentified |
| 99128.097 | C6H- 36-35 |
| 99134.046 | C6H 21/2 J=71/2-69/2 e |
| 99143.725 | g-CH3CH2OH 27(2,26)-27(1,26) t=1-0 |
| 99172.534 | CH3CH213CN 11(2,9)-10(2,8) |
| 99203.460 | CH3SH 2(1)-2(0) E |
| 99264.980 | CH3SH 3(1)-3(0) E |
| 99299.905 | SO N,J=2,3-1,2 |
| 99311.195 | NH2CN 5(1,5)-4(1,4) |
| 99324.358 | CH3OCH3 4(1,4)-3(0,3) EA+AE |
| 99325.208 | CH3OCH3 4(1,4)-3(0,3) EE |
| 99326.058 | CH3OCH3 4(1,4)-3(0,3) AA |
| 99361.000 | unidentified |
| 99378.000 | unidentified |
| 99392.526 | SO2 29(4,26)-28(5,23) |
Spectral window: 2
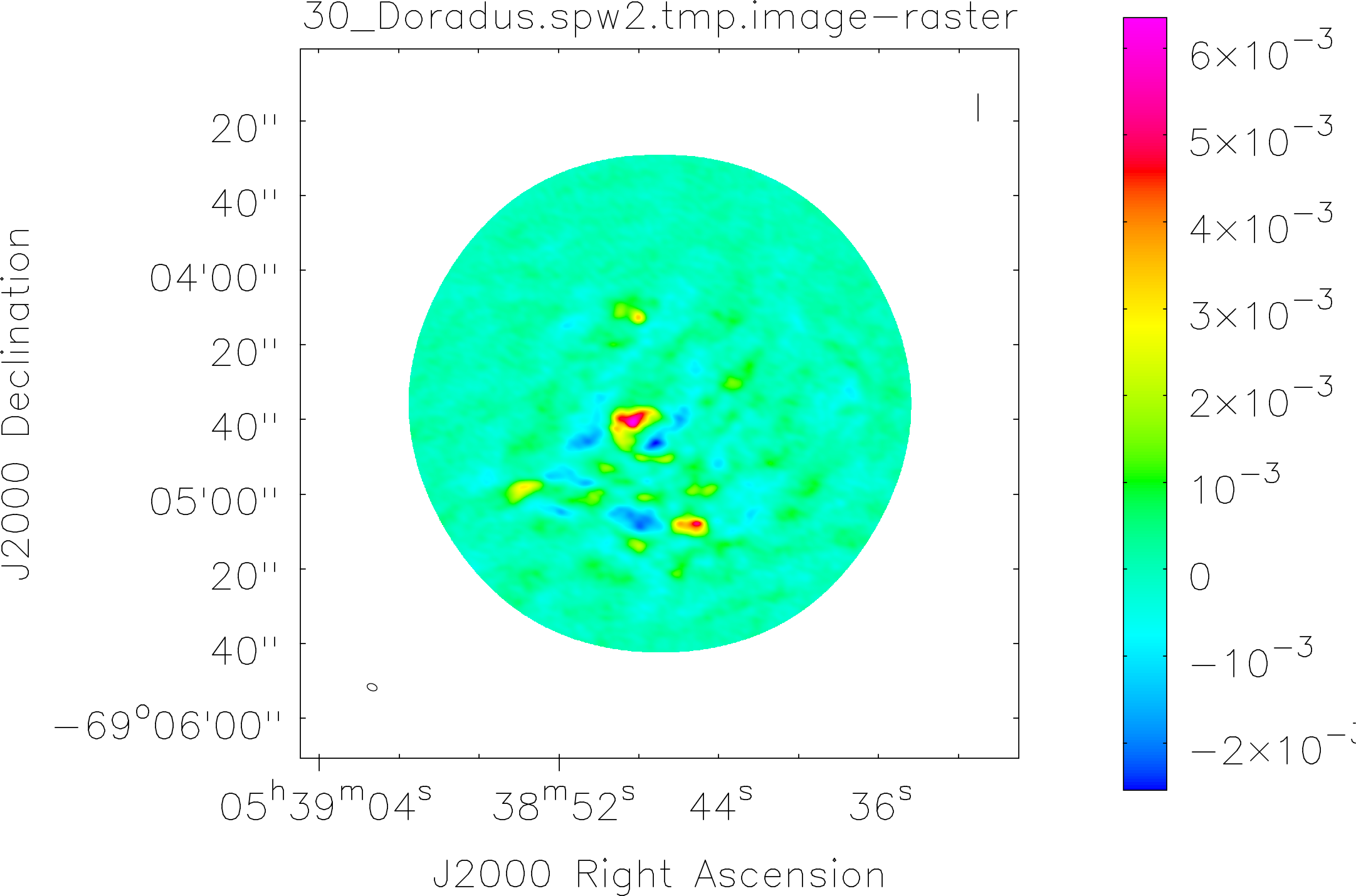
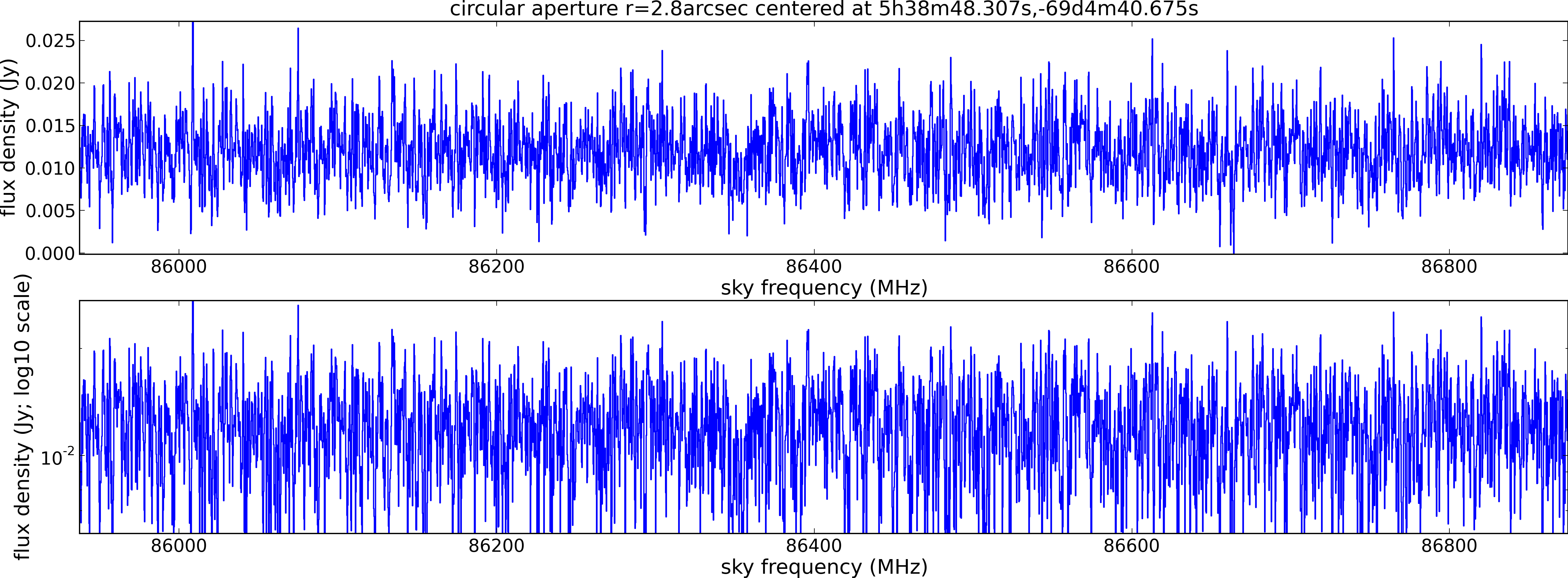
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 85939.100 | unidentified |
| 85943.000 | unidentified |
| 85945.968 | CH2CHCN 9(8,*)-8(8,*) 2v11 |
| 85973.249 | CH3OCH3 13(2,12)-12(3,9) AA |
| 85976.131 | CH3OCH3 13(2,12)-12(3,9) EE |
| 85979.002 | CH3OCH3 13(2,12)-12(3,9) EA |
| 85979.025 | CH3OCH3 13(2,12)-12(3,9) AE |
| 85987.176 | CH2CHCN 9(2,7)-8(2,6) 1v11 |
| 86010.000 | unidentified |
| 86021.008 | CH3OCHO 7(5,2)-6(5,1) E |
| 86027.674 | CH3OCHO 7(5,3)-6(5,2) E |
| 86029.445 | CH3OCHO 7(5,3)-6(5,2) A |
| 86030.212 | CH3OCHO 7(5,2)-6(5,1) A |
| 86034.300 | unidentified |
| 86048.500 | C4H 2S J=9-8 2v7 L |
| 86054.967 | HC15N 1-0 |
| 86074.274 | CH3NH2 4(1)A2 -4(0)A1 F=3-3 |
| 86074.498 | CH3NH2 4(1)A2 -4(0)A1 F=5-5 |
| 86075.369 | CH3NH2 4(1)A2 -4(0)A1 F=4-4 |
| 86093.983 | SO N,J=2,2-1,1 |
| 86104.440 | C4H 2S J=9-8 2v7 U |
| 86133.200 | unidentified |
| 86148.000 | unidentified |
| 86151.600 | unidentified |
| 86181.413 | CCS N,J=7,6-6,5 |
| 86204.600 | unidentified |
| 86207.800 | unidentified |
| 86210.079 | CH3OCHO 7(4,4)-6(4,3) A |
| 86220.900 | unidentified |
| 86223.548 | CH3OCHO 7(4,3)-6(4,2) E |
| 86223.780 | CH3OCH3 2(2,0)-2(1,1) AE |
| 86224.106 | CH3OCHO 7(4,4)-6(4,3) E |
| 86225.615 | CH3OCH3 2(2,0)-2(1,1) EA |
| 86226.727 | CH3OCH3 2(2,0)-2(1,1) EE |
| 86228.720 | CH3OCH3 2(2,0)-2(1,1) AA |
| 86239.600 | unidentified |
| 86243.430 | SiO 2-1 v=1 |
| 86243.500 | unidentified |
| 86248.200 | unidentified |
| 86250.576 | CH3OCHO 7(4,3)-6(4,2) A |
| 86254.848 | CH2CHCN 9(2,7)-8(2,6) 2v11 |
| 86259.700 | unidentified |
| 86263.100 | unidentified |
| 86263.500 | unidentified |
| 86265.826 | CH3OCHO 7(3,5)-6(3,4) A |
| 86268.659 | CH3OCHO 7(3,5)-6(3,4) E |
| 86291.800 | unidentified |
| 86297.200 | unidentified |
| 86300.500 | unidentified |
| 86300.700 | unidentified |
| 86311.267 | g-CH3CH2OH 5(2,4)-4(2,3) t=0-0 |
| 86312.700 | unidentified |
| 86317.000 | unidentified |
| 86338.736 | H13CN 1-0 F=1-1 |
| 86340.176 | H13CN 1-0 F=2-1 |
| 86342.255 | H13CN 1-0 F=0-1 |
| 86369.100 | unidentified |
| 86378.200 | unidentified |
| 86381.831 | NH2CHO 7(1,6)-7(0,7) F=7-7 |
| 86382.751 | NH2CHO 7(1,6)-7(0,7) |
| 86383.130 | NH2CHO 7(1,6)-7(0,7) F=8-8 |
| 86383.318 | NH2CHO 7(1,6)-7(0,7) F=6-6 |
| 86386.300 | unidentified |
| 86389.200 | unidentified |
| 86389.200 | unidentified |
| 86395.800 | unidentified |
| 86398.474 | CH3CH213CN 10(1,10)-9(1,9) |
| 86398.600 | unidentified |
| 86398.800 | unidentified |
| 86416.900 | unidentified |
| 86421.800 | unidentified |
| 86440.200 | unidentified |
| 86445.800 | unidentified |
| 86458.271 | CH2DCN 5(1,5)-4(1,4) |
| 86459.300 | unidentified |
| 86473.000 | unidentified |
| 86473.400 | unidentified |
| 86481.000 | unidentified |
| 86484.212 | CH3CH2CN 29(3,27)-28(4,24) |
| 86486.600 | unidentified |
| 86492.970 | HCOOD 4(0,4)-3(0,3) |
| 86536.600 | unidentified |
| 86543.700 | unidentified |
| 86546.180 | HCOOH 4(1,4)-3(1,3) |
| 86555.912 | g-CH3CH2OH 5(4,2)-4(4,1) t=0-0 |
| 86556.012 | g-CH3CH2OH 5(4,1)-4(4,0) t=0-0 |
| 86557.564 | s-CH2CHOH 2(1,2)-1(0,1) |
| 86562.780 | Si13CC 4(1,4)-3(1,3) |
| 86570.249 | C7H 21/2 99/2-97/2 f |
| 86593.687 | CCCO 9-8 |
| 86604.292 | g-CH3CH2OH 5(3,3)-4(3,2) t=0-0 |
| 86615.602 | CH3OH 7(2,6)-6(3,3) A-- |
| 86617.742 | 29Si34S 5-4 |
| 86621.734 | g-CH3CH2OH 5(3,2)-4(3,2) t=0-0 |
| 86639.095 | SO2 8(3,5)-9(2,8) |
| 86650.849 | CH2CHCH3 5(0,5)-4(0,4) E |
| 86651.584 | CH2CHCH3 5(0,5)-4(0,4) A |
| 86670.820 | HCO 1(0,1)-0(0,0) 3/2-1/2 F=2-1 |
| 86708.350 | HCO 1(0,1)-0(0,0) 3/2-1/2 F=1-0 |
| 86708.374 | CCCS 15-14 |
| 86745.317 | CH3CH2CN 8(1,8)-7(0,7) |
| 86754.288 | H13CO+ 1-0 |
| 86777.430 | HCO 1(0,1)-0(0,0) 1/2-1/2 F=1-1 |
| 86781.400 | unidentified |
| 86784.500 | unidentified |
| 86794.500 | unidentified |
| 86803.600 | unidentified |
| 86805.750 | HCO 1(0,1)-0(0,0) 1/2-1/2 F=0-1 |
| 86812.300 | unidentified |
| 86814.388 | CH2DCN 5(4,*)-4(4,*) |
| 86819.848 | CH3CH2CN 10(1,10)-9(1,9) |
| 86824.595 | CH2DCN 5(3,3)-4(3,2) |
| 86824.597 | CH2DCN 5(3,2)-4(3,1) |
| 86831.480 | CH3SH 13(3)-14(2) A++ |
| 86833.932 | CH2DCN 5(0,5)-4(0,4) |
| 86846.995 | SiO 2-1 v=0 |
| 86864.000 | unidentified |
Spectral window: 3
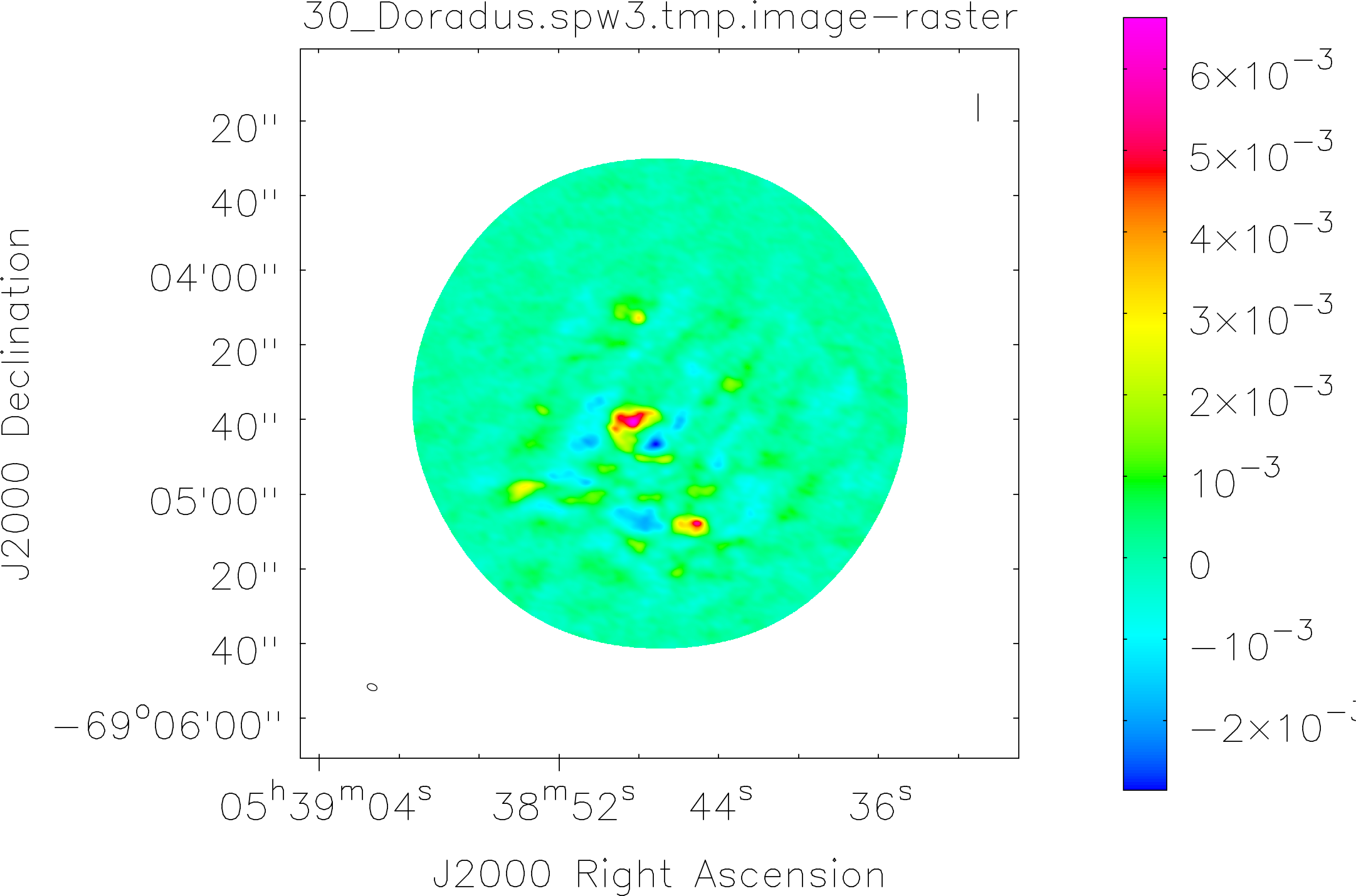
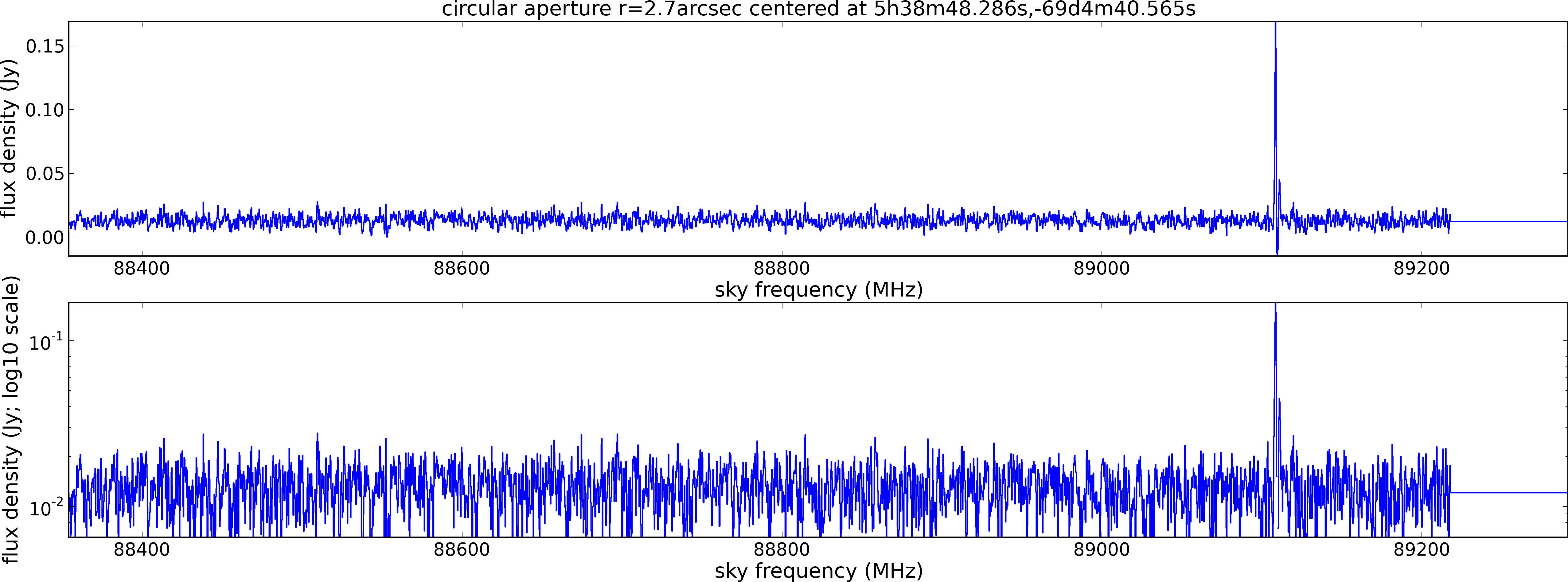
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 88358.420 | CH3OCHO 22(5,17)-22(4,18) A |
| 88402.000 | unidentified |
| 88445.000 | unidentified |
| 88481.000 | unidentified |
| 88540.600 | unidentified |
| 88594.809 | CH3OH 15(3,13)-14(4,10) A++ |
| 88630.415 | HCN 1-0 F=1-1 |
| 88631.847 | HCN 1-0 F=2-1 |
| 88633.936 | HCN 1-0 F=0-1 |
| 88667.901 | CH3NH2 2(0)B1 -1(0)B2 |
| 88668.681 | CH3NH2 2(0)E2+1 -1(0)E2+1 |
| 88669.543 | CH3NH2 2(0)E1+1 -1(0)E1+1 |
| 88669.626 | CH3NH2 2(0)A1 -1(0)A2 |
| 88706.220 | CH3OCH3 15(2,13)-15(1,14) EA+AE |
| 88707.701 | CH3OCH3 15(2,13)-15(1,14) EE |
| 88709.181 | CH3OCH3 15(2,13)-15(1,14) AA |
| 88720.567 | 34SO2 7(3,5)-8(2,6) |
| 88723.239 | CH3OCHO 11(3,9)-11(2,10) A |
| 88741.800 | unidentified |
| 88749.800 | unidentified |
| 88758.419 | CH3CH2CN 27(3,24)-27(2,25) |
| 88765.500 | unidentified |
| 88770.800 | unidentified |
| 88843.117 | CH3OCHO 7(1,6)-6(1,5) E |
| 88851.641 | CH3OCHO 7(1,6)-6(1,5) A |
| 88855.080 | CH3CH213CN 10(2,9)-9(2,8) |
| 88861.000 | unidentified |
| 88865.692 | H15NC 1-0 |
| 88913.850 | C5H 23/2 J=37/2-35/2 f |
| 88915.940 | C5H 23/2 J=37/2-35/2 e |
| 88916.000 | unidentified |
| 88939.993 | CH3OH 15(3,12)-14(4,11) A-- |
| 88940.238 | H2CCCC 10(1,10)-9(1,9) |
| 88957.000 | unidentified |
| 88977.000 | unidentified |
| 89043.500 | unidentified |
| 89045.590 | CCCN 9-8 J=19/2-17/2 |
| 89060.827 | t-CH3CH2OH 18(4,14)-17(5,13) |
| 89064.360 | CCCN 9-8 J=17/2-15/2 |
| 89082.200 | unidentified |
| 89084.000 | unidentified |
| 89086.423 | HCN 1-0 F=1-1 (0,2,0)=0 |
| 89087.914 | HCN 1-0 F=2-1 (0,2,0)=0 |
| 89090.130 | HCN 1-0 F=0-1 (0,2,0)=0 |
| 89093.200 | unidentified |
| 89103.720 | 29SiS 5-4 |
| 89104.300 | HC7N 79-78 |
| 89116.025 | CH3CH213CN 10(6,*)-9(6,*) |
| 89118.949 | CH3CH213CN 10(7,*)-9(7,*) |
| 89121.504 | CH3CH213CN 10(5,*)-9(5,*) |
| 89122.297 | CH313CH2CN 10(6,*)-9(6,*) |
| 89123.590 | CH313CH2CN 10(7,*)-9(7,*) |
| 89129.913 | CH313CH2CN 10(5,*)-9(5,*) |
| 89130.350 | CH313CH2CN 10(8,*)-9(8,*) |
| 89142.905 | CH3CH213CN 10(4,7)-9(4,6) |
| 89143.857 | CH3CH213CN 10(4,6)-9(4,5) |
| 89154.617 | CH313CH2CN 10(4,7)-9(4,6) |
| 89155.789 | CH313CH2CN 10(4,6)-9(4,5) |
| 89188.526 | HCO+ 1-0 |
| 89234.000 | unidentified |
| 89235.656 | CH3CH213CN 10(3,7)-9(3,6) |
| 89251.160 | CH2DOH 2(0,2)-1(0,1) o1 |
| 89259.143 | CH313CH2CN 10(3,7)-9(3,6) |
| 89275.410 | CH2DOH 2(0,2)-1(0,1) e1 |
uv sampling
| FieldID | SpwID | uv_min(klambda) | uv_max(klambda) |
|---|---|---|---|
| 0 | 0 | 25.009 | 361.357 |
| 0 | 1 | 25.009 | 361.356 |
| 0 | 2 | 25.012 | 361.334 |
| 0 | 3 | 25.009 | 361.351 |
| 1 | 0 | 25.404 | 353.677 |
| 1 | 1 | 25.400 | 353.666 |
| 1 | 2 | 25.419 | 353.620 |
| 1 | 3 | 25.407 | 353.653 |
| 3 | 0 | 24.348 | 372.823 |
| 3 | 1 | 24.344 | 372.795 |
| 3 | 2 | 24.339 | 372.752 |
| 3 | 3 | 24.350 | 373.029 |
| 4 | 0 | 23.166 | 360.704 |
| 4 | 1 | 23.160 | 360.702 |
| 4 | 2 | 23.198 | 360.647 |
| 4 | 3 | 23.178 | 360.553 |
| 5 | 0 | 21.546 | 366.897 |
| 5 | 1 | 21.547 | 366.840 |
| 5 | 2 | 21.549 | 366.826 |
| 5 | 3 | 21.545 | 366.964 |
| 6 | 0 | 21.522 | 355.379 |
| 6 | 1 | 21.523 | 355.381 |
| 6 | 2 | 21.526 | 355.382 |
| 6 | 3 | 21.521 | 355.365 |
| 7 | 0 | 23.081 | 360.991 |
| 7 | 1 | 23.075 | 360.991 |
| 7 | 2 | 23.113 | 360.990 |
| 7 | 3 | 23.092 | 360.993 |
| 8 | 0 | 21.040 | 372.926 |
| 8 | 1 | 21.040 | 372.906 |
| 8 | 2 | 21.041 | 372.875 |
| 8 | 3 | 21.039 | 373.075 |
| 9 | 0 | 21.200 | 361.505 |
| 9 | 1 | 21.201 | 361.505 |
| 9 | 2 | 21.202 | 361.505 |
| 9 | 3 | 21.200 | 361.507 |