2011.0.00061.S/2011.0.00061.S_2012-12-25/2011.0.00061.S/sg_ouss_id/group_ouss_id/member_ouss_2012-12-25_id/calibrated
full summary of this dataset can be found here
Spectral setup
| SpwID | Frame | #chan | skyfreq(MHz) | Chwid(kHz) | BW(MHz) | beam("x" deg) |
|---|---|---|---|---|---|---|
| 0 | TOPO | 3840 | 96375.0 ~ 98249.5 | -488.281 | 1875.000000 | 2.4 x 1.6, 82.6 |
| 1 | TOPO | 3840 | 98374.0 ~ 100248.5 | -488.281 | 1875.000000 | 2.4 x 1.6, 82.8 |
| 2 | TOPO | 3840 | 108417.0 ~ 110291.5 | 488.281 | 1875.000000 | 2.2 x 1.5, 82.8 |
| 3 | TOPO | 3840 | 109417.0 ~ 111291.5 | 488.281 | 1875.000000 | 2.1 x 1.5, 83.0 |
Observed fields
| FieldID | FieldName | R.A. | Dec | Epoch | nRows |
|---|---|---|---|---|---|
| 0 | J2258-279 | 22:58:05.960000 | -27.58.21.26000 | J2000 | 71712 |
| 2 | Uranus | 00:30:45.625820 | +02.30.49.29445 | J2000 | 35712 |
| 3 | J0132-169 | 01:32:43.472260 | -16.54.48.49793 | J2000 | 72000 |
| 4 | NGC253 | 00:47:33.300000 | -25.17.23.00000 | J2000 | 596080 |
| 5 | Uranus | 00:30:40.685818 | +02.30.16.07938 | J2000 | 35724 |
Spectral window: 0
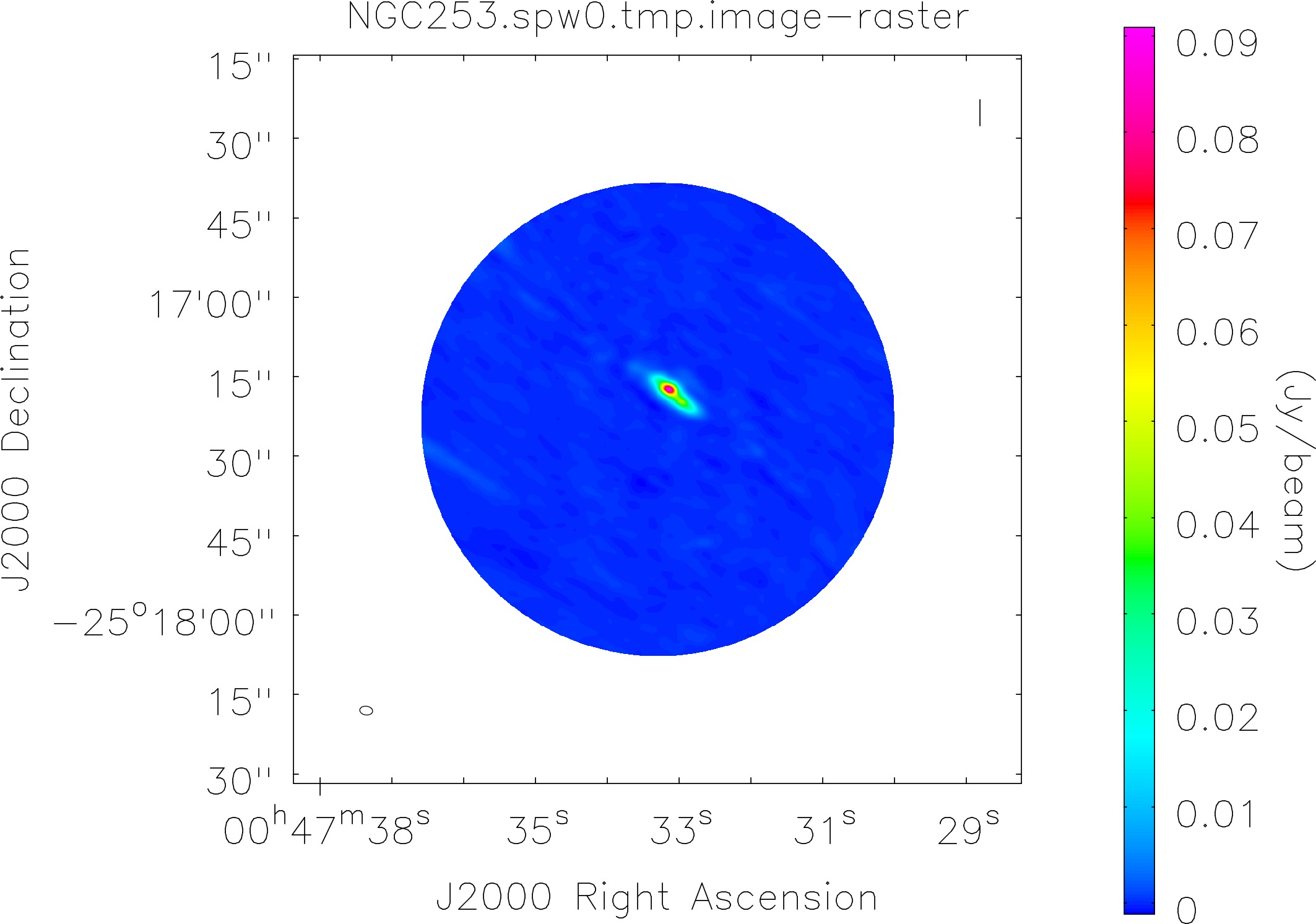
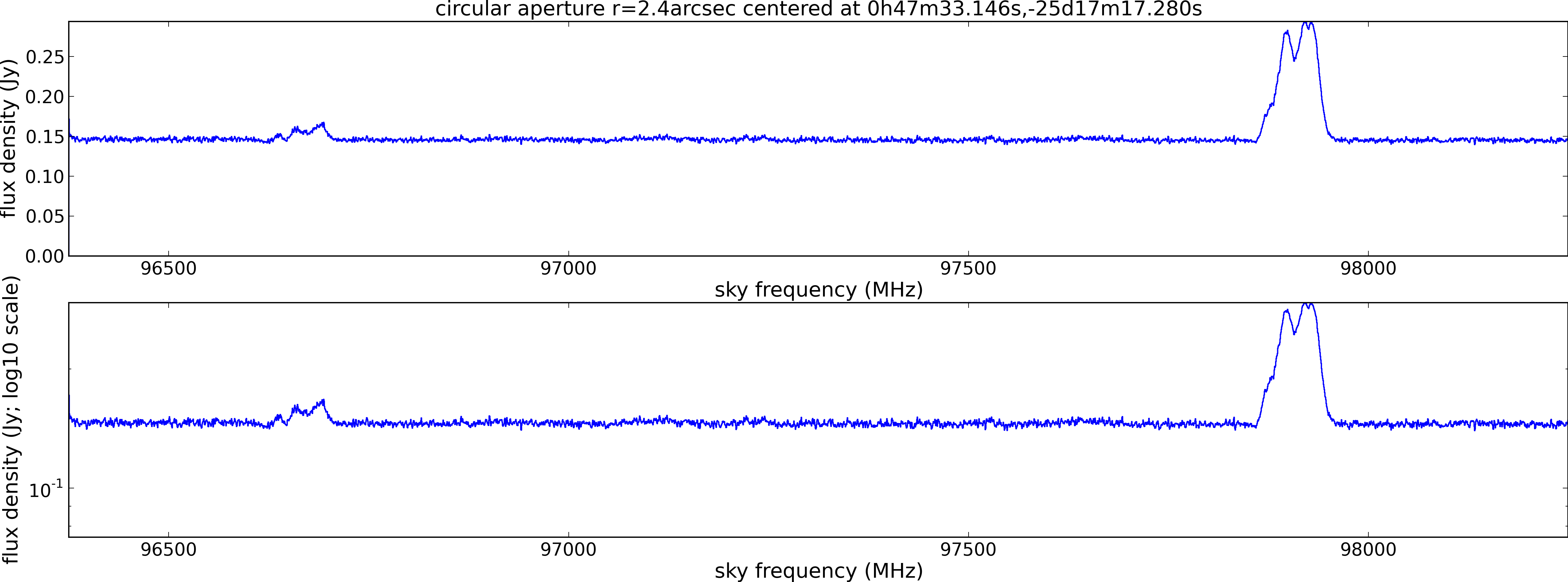
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 96384.417 | CH3CHO 5(-3,3) - 4(-3,2) E |
| 96391.200 | unidentified |
| 96396.055 | CH3OH 2(1,2)-1(1,1) A++ t=1 |
| 96412.950 | C34S 2-1 |
| 96425.620 | CH3CHO 5(-2,4) - 4(-2,3) E |
| 96437.000 | unidentified |
| 96452.607 | CH3CH213CN 11(0,11)-10(0,10) |
| 96475.523 | CH3CHO 5(2,3) - 4(2,2) E |
| 96478.300 | C4H 23/2 J=21/2-19/2 1v7e |
| 96492.164 | CH3OH 2(1,2)-1(1,1) E t=1 |
| 96493.553 | CH3OH 2(0,2)-1(0,1) E t=1 |
| 96501.698 | CH3OH 2(-1,1)-1(-1,0) E t=1 |
| 96513.671 | CH3OH 2(0,2)-1(0,1) A++ t=1 |
| 96536.802 | CH2CHCN 31(5,27)-32(4,28) |
| 96588.593 | CH3OH 2(1,1)-1(1,0) A-- t=1 |
| 96613.156 | CH3OCHO 8(4,5)-8(3,6) E |
| 96632.668 | CH3CHO 5(2,3) - 4(2,2) A++ |
| 96637.769 | CH3OCHO 7(4,4)-7(3,5) A |
| 96648.099 | CH3OCHO 5(4,1)-5(3,2) E |
| 96670.896 | CH3OCHO 5(4,2)-5(3,3) E |
| 96676.820 | HCCCCN 21(20)-20(19) |
| 96691.570 | CH2DCCH 6(1,6)-5(1,5) |
| 96693.517 | CH3OCHO 6(4,3)-6(3,4) A |
| 96696.390 | HCCCCN 21(21)-20(20) |
| 96709.210 | CH3OCHO 8(4,5)-8(3,6) A |
| 96711.880 | HCCCCN 21(22)-20(21) |
| 96720.000 | unidentified |
| 96739.363 | CH3OH 2(-1,2)-1(-1,1) E |
| 96741.377 | CH3OH 2(0,2)_1(0,1) A++ |
| 96744.549 | CH3OH 2(0,2)-1(0,1) E |
| 96755.507 | CH3OH 2(1,1)-1(1,0) E |
| 96775.000 | unidentified |
| 96781.827 | 34SO N,J=5,4-4,4 |
| 96797.000 | unidentified |
| 96822.000 | unidentified |
| 96847.241 | CH3OCH3 5(2,4)-5(1,5) AE |
| 96847.241 | CH3OCH3 5(2,4)-5(1,5) EA |
| 96849.881 | CH3OCH3 5(2,4)-5(1,5) EE |
| 96852.496 | CH3OCH3 5(2,4)-5(1,5) AA |
| 96919.754 | CH3CH2CN 11(0,11)-10(0,10) |
| 96988.123 | O13CS 8-7 |
| 97029.693 | CCCN- 10-9 |
| 97069.000 | unidentified |
| 97080.695 | CH2DCCH 6(0,6)-5(0,5) |
| 97169.513 | C33S 2-1 3/2-3/2 |
| 97171.840 | C33S 2-1 1/2-1/2 |
| 97171.840 | C33S 2-1 7/2-5/2 + 5/2-3/2 |
| 97174.996 | C33S 2-1 5/2-5/2 |
| 97175.271 | C33S 2-1 3/2-1/2 |
| 97218.353 | CH2CHCHO 11(2,10)-10(2,9) |
| 97229.010 | 13CH3CH2CN 11(2,9)-10(2,8) |
| 97244.700 | C4H 2D5/2 J=21/2-19/2 2v7 =2 |
| 97263.540 | g-CH3CH2OH 23(0,23)-23(1,23) t=1-0 |
| 97270.996 | CS 2-1 v=1 |
| 97276.000 | unidentified |
| 97282.000 | unidentified |
| 97286.824 | CH2CHCN 6(1,6)-5(0,5) |
| 97294.123 | CH3OCH3 28(7,21)-27(8,19) EE |
| 97295.480 | Si13CC 4(1,3)-3(1,2) |
| 97301.208 | OCS 8-7 |
| 97318.571 | CH3OCHO 4(2,2)-3(1,3) E |
| 97535.908 | g-CH3CH2OH 21(1,21)-21(0,21) t=1-0 |
| 97536.849 | g-CH3CH2OH 23(1,23)-23(0,23) t=1-0 |
| 97546.875 | g-CH3CH2OH 29(1,28)-29(2,28) t=1-0 |
| 97549.692 | g-CH3CH2OH 26(0,26)-26(1,26) t=1-0 |
| 97562.844 | g-CH3CH2OH 24(1,24)-24(0,24) t=1-0 |
| 97569.000 | unidentified |
| 97574.042 | g-CH3CH2OH 20(1,20)-20(0,20) t=1-0 |
| 97577.278 | CH3OCHO 8(5,3)-7(5,2) E t=1 |
| 97577.900 | unidentified |
| 97582.808 | CH3OH 2(1,1)-1(1,0) A-- |
| 97597.180 | CH3OCHO 8(3,6)-7(3,5) A t=1 |
| 97600.390 | g-CH3CH2OH 25(1,25)-25(0,25) t=1-0 |
| 97603.000 | unidentified |
| 97618.700 | unidentified |
| 97631.329 | g-CH3CH2OH 27(0,27)-27(1,27) t=1-0 |
| 97632.700 | H213CS 3(1,3)-2(1,2) |
| 97649.502 | g-CH3CH2OH 19(1,19)-19(0,19) t=1-0 |
| 97651.392 | CH3OCHO 10(4,7)-10(3,8) E |
| 97661.356 | CH3OCHO 8(4,5)-7(4,4) A t=1 |
| 97662.000 | unidentified |
| 97672.030 | CH313CH2CN 11(2,10)-10(2,9) |
| 97678.260 | CH3OH 21(6,16)-22(5,17) A-- |
| 97679.380 | CH3OH 21(6,15)-22(5,18) A++ |
| 97694.197 | CH3OCHO 10(4,7)-10(3,8) A |
| 97702.340 | SO2 7(3,5)-8(2,6) |
| 97715.401 | 34SO N,J=2,3-1,2 |
| 97729.400 | unidentified |
| 97738.762 | CH3OCHO 8(6,3)-7(6,2) E t=1 |
| 97739.300 | unidentified |
| 97752.824 | CH3OCHO 8(4,4)-7(4,3) A t=1 |
| 97753.400 | unidentified |
| 97755.610 | g-CH3CH2OH 28(1,28)-28(0,28) t=1-0 |
| 97774.307 | g-CH3CH2OH 18(1,18)-18(0,18) t=1-0 |
| 97815.987 | g-CH3CH2OH 29(1,29)-29(0,20) t=1-0 |
| 97833.634 | H2CCCC 11(1,11)-10(1,10) |
| 97846.300 | unidentified |
| 97862.540 | C5H 21/2 J=41/2-39/2 e |
| 97868.730 | C5H 21/2 J=41/2-39/2 f |
| 97869.800 | unidentified |
| 97874.000 | unidentified |
| 97885.626 | CH3OCHO 8(5,4)-7(5,3) E t=1 |
| 97886.000 | unidentified |
| 97897.129 | CH3OCHO 8(4,4)-7(4,3) E t=1 |
| 97897.500 | unidentified |
| 97915.600 | unidentified |
| 97926.000 | unidentified |
| 97931.200 | unidentified |
| 97932.445 | g-CH3CH2OH 31(0,31)-31(1,31) t=1-0 |
| 97957.200 | unidentified |
| 97962.858 | g-CH3CH2OH 17(1,17)-17(0,17) t=1-0 |
| 97980.953 | CS 2-1 |
| 97995.212 | l-C3H 21/2 J=9/2-7/2 F=5-4e |
| 97995.951 | l-C3H 21/2 J=9/2-7/2 F=4-3e |
| 98011.649 | l-C3H 21/2 J=9/2-7/2 F=5-4f |
| 98012.576 | l-C3H 21/2 J=9/2-7/2 F=4-3f |
| 98030.400 | CH3OH 24(6,19)-23(7,16) A-- |
| 98030.440 | CH3OH 24(6,18)-23(7,17) A++ |
| 98033.907 | CH3CH213CN 11(7,*)-10(7,*) |
| 98039.612 | CH313CH2CN 11(7,*)-10(7,*) |
| 98040.565 | CH313CH2CN 11(6,*)-10(6,*) |
| 98042.563 | CH3CH213CN 11(5,*)-10(5,*) |
| 98052.969 | CH313CH2CN 11(5,*)-10(5,*) |
| 98056.191 | CH313CH2CN 11(9,*)-10(9,*) |
| 98060.000 | unidentified |
| 98072.727 | CH3CH213CN 11(4,8)-10(4,7) |
| 98074.628 | CH3CH213CN 11(4,7)-10(4,6) |
| 98087.345 | CH313CH2CN 11(4,8)-10(4,7) |
| 98089.684 | CH313CH2CN 11(4,7)-10(4,6) |
| 98117.426 | CH3CH213CN 11(3,9)-10(3,8) |
| 98134.865 | CH313CH2CN 11(3,9)-10(3,8) |
| 98153.787 | SiC4 32-31 |
| 98165.346 | 13CH3CH2CN 11(1,10)-10(1,9) |
| 98177.578 | CH3CH2CN 11(2,10)-10(2,9) |
| 98182.199 | CH3OCHO 8(7,1)-7(7,0) E |
| 98190.653 | CH3OCHO 8(7,1)-7(7,0) A |
| 98190.653 | CH3OCHO 8(7,2)-7(7,1) A |
| 98191.414 | CH3OCHO 8(7,2)-7(7,1) E |
| 98218.353 | H2CCCC 11(3,9)-10(3,8) |
| 98218.355 | H2CCCC 11(3,8)-10(3,7) |
| 98230.313 | g-CH3CH2OH 16(1,16)-16(0,16) t=1-0 |
| 98238.285 | H2CCCC 11(2,9)-10(2,8) |
| 98244.941 | H2CCCC 11(0,11)-10(0,10) |
Spectral window: 1
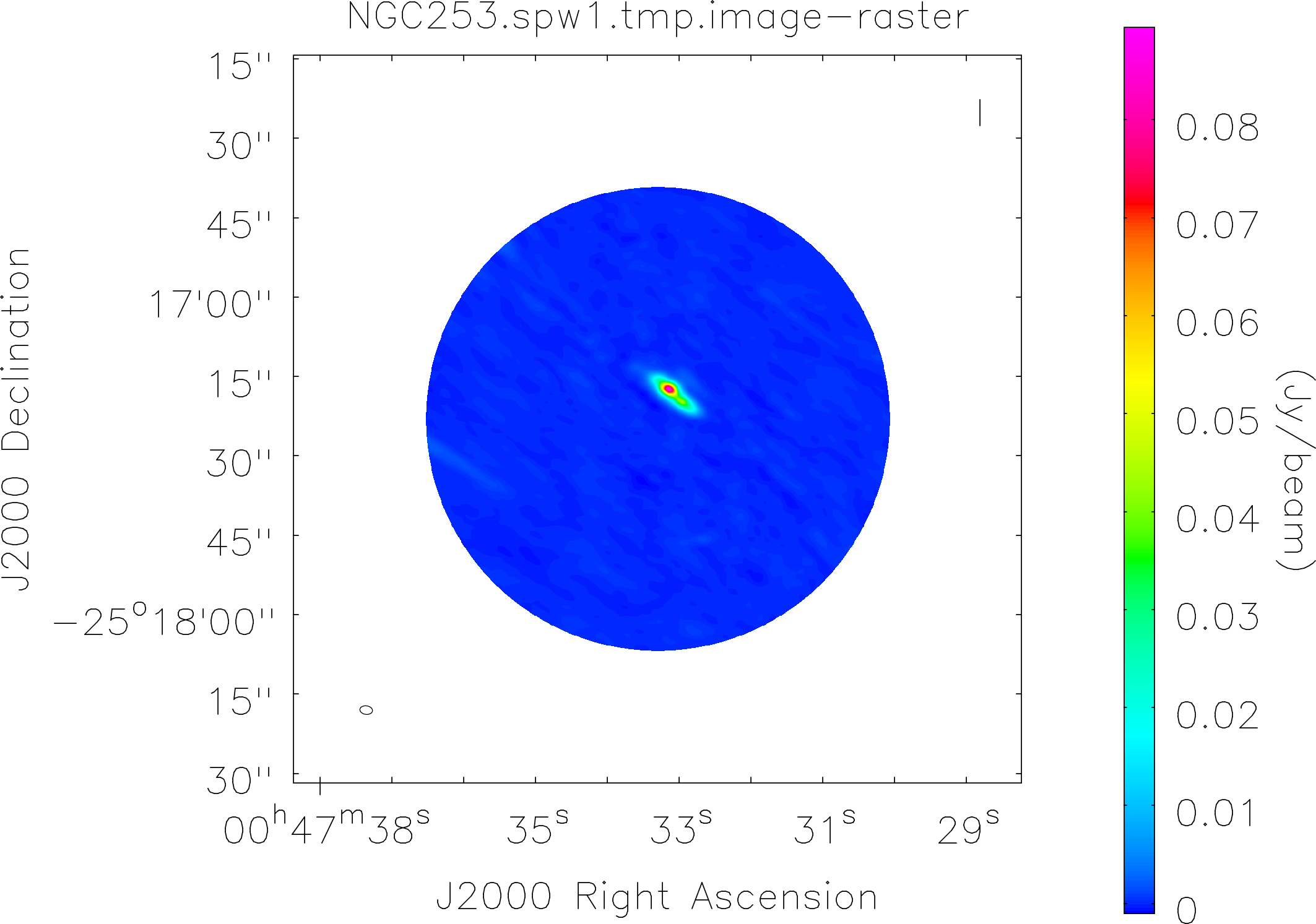
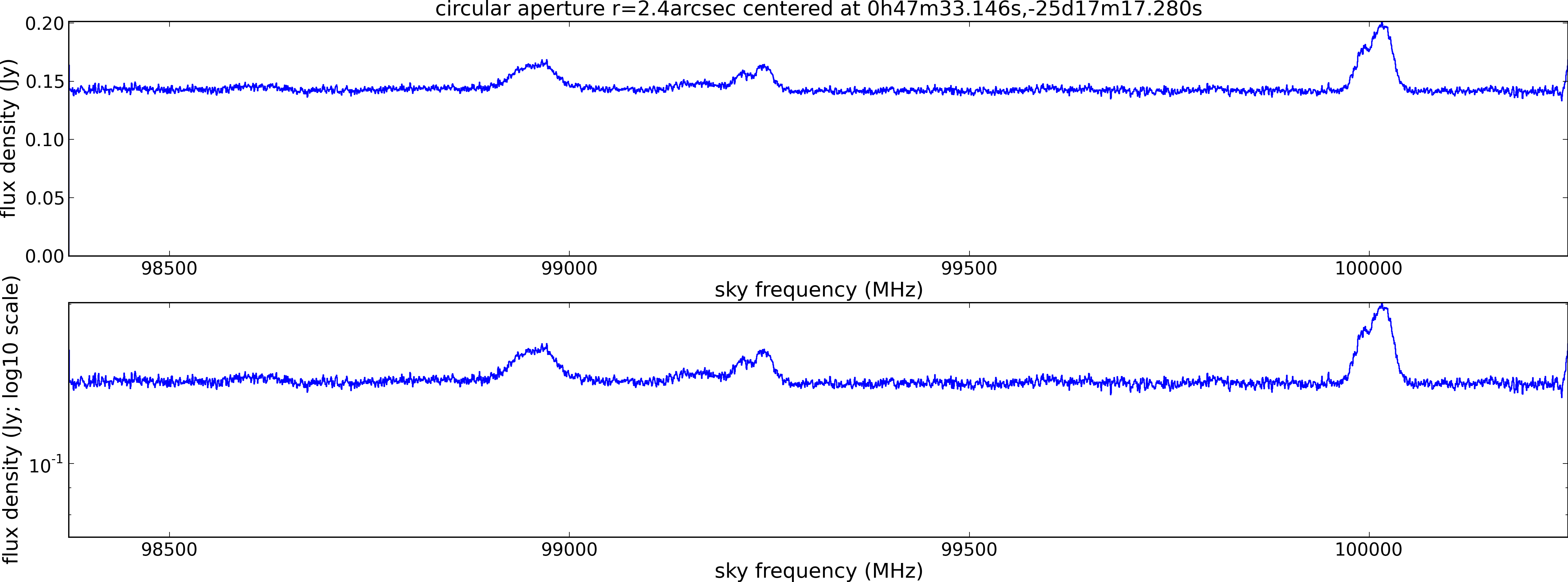
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 98408.606 | C6H 23/2 J=71/2-69/2 e |
| 98424.082 | CH3OCHO 8(5,3)-7(5,2) E |
| 98431.748 | CH3OCHO 8(5,4)-7(5,3) E |
| 98432.773 | CH3OCHO 8(5,4)-7(5,3) A |
| 98435.820 | CH3OCHO 8(5,3)-7(5,2) A |
| 98441.808 | C6H 23/2 J=71/2-69/2 f |
| 98474.550 | 33SO N,J=2,3-1,2 F=3/2-1/2 |
| 98482.150 | 33SO N,J=2,3-1,2 F=5/2-3/2 |
| 98489.080 | 33SO N,J=2,3-1,2 F=7/2-5/2 |
| 98493.680 | 33SO N,J=2,3-1,2 F=9/2-7/2 |
| 98512.519 | HC5N 37-36 |
| 98523.881 | CH3CH2CN 11(6)-10(6) |
| 98524.663 | CH3CH2CN 11(7)-10(7) |
| 98524.690 | C5H 23/2 J=41/2-39/2 f |
| 98527.190 | C5H 23/2 J=41/2-39/2 e |
| 98532.075 | CH3CH2CN 11(8)-10(8) |
| 98533.983 | CH3CH2CN 11(5)-10(5) |
| 98544.152 | CH3CH2CN 11(9,*)-10(9,*) |
| 98564.832 | CH3CH2CN 11(4,8)-10(4,7) |
| 98566.797 | CH3CH2CN 11(4,7)-10(4,6) |
| 98606.771 | CH3OCHO 8(3,6)-7(3,5) E |
| 98610.104 | CH3CH2CN 11(3,9)-10(3,8) |
| 98611.195 | CH3OCHO 8(3,6)-7(3,5) A |
| 98630.000 | unidentified |
| 98651.514 | (CH3)2CO 5(5,1)-4(4,1) EE |
| 98655.097 | H2CCCC 11(1,10)-10(1,9) |
| 98663.000 | unidentified |
| 98682.635 | CH3OCHO 8(4,5)-7(4,4) A |
| 98696.000 | unidentified |
| 98701.106 | CH3CH2CN 11(3,8)-10(3,7) |
| 98711.931 | CH3OCHO 8(4,5)-7(4,4) E |
| 98747.797 | CH3OCHO 8(4,4)-7(4,3) E |
| 98771.000 | unidentified |
| 98792.314 | CH3OCHO 8(4,4)-7(4,3) A |
| 98800.980 | (CH3)2CO 5(5,0)-4(4,0) EE |
| 98863.314 | CH3CHO 5(1,4) - 4(1,3) E |
| 98875.160 | CH3OCHO 11(4,8)-11(3,9) A |
| 98900.951 | CH3CHO 5(1,4) - 4(1,3) A-- |
| 98926.723 | AlF 3-2 |
| 98940.020 | CCCN 10-9 J=21/2-19/2 |
| 98958.780 | CCCN 10-9 J=19/2-17/2 |
| 98976.278 | SO2 28(7,21)-29(6,24) |
| 99011.000 | unidentified |
| 99068.000 | unidentified |
| 99083.405 | C6H 21/2 J=71/2-69/2 f |
| 99087.000 | unidentified |
| 99118.600 | NH2D 5(2,4)-4(1,4) |
| 99120.000 | unidentified |
| 99128.097 | C6H- 36-35 |
| 99134.046 | C6H 21/2 J=71/2-69/2 e |
| 99143.725 | g-CH3CH2OH 27(2,26)-27(1,26) t=1-0 |
| 99172.534 | CH3CH213CN 11(2,9)-10(2,8) |
| 99203.460 | CH3SH 2(1)-2(0) E |
| 99264.980 | CH3SH 3(1)-3(0) E |
| 99299.905 | SO N,J=2,3-1,2 |
| 99311.195 | NH2CN 5(1,5)-4(1,4) |
| 99324.358 | CH3OCH3 4(1,4)-3(0,3) EA+AE |
| 99325.208 | CH3OCH3 4(1,4)-3(0,3) EE |
| 99326.058 | CH3OCH3 4(1,4)-3(0,3) AA |
| 99361.000 | unidentified |
| 99378.000 | unidentified |
| 99392.526 | SO2 29(4,26)-28(5,23) |
| 99409.740 | CH3SH 4(1)-4(0) E |
| 99422.080 | (CH3)2CO 14(*,11)-14(*,12) EE |
| 99586.000 | unidentified |
| 99651.863 | HC13CCN 11-10 |
| 99661.471 | HCC13CN 11-10 |
| 99672.230 | CH2DOH 6(1,5)-6(0,6) |
| 99681.511 | CH3CH2CN 11(2,9)-10(2,8) |
| 99689.294 | CH2CN 5(1,5)-4(1,4) J=11/2-9/2 |
| 99727.000 | unidentified |
| 99730.959 | CH3OH 6(1,6)-5(0,5) E t=1 |
| 99774.130 | H2C34S 3(1,3)-2(1,2) |
| 99866.509 | CCS N,J=8,7-7,6 |
| 99903.000 | unidentified |
| 99929.540 | K35Cl 13-12 |
| 99953.270 | NH2CN 5(2,4)-4(2,3) |
| 99956.600 | NH2CN 5(2,3)-4(2,2) |
| 99972.660 | NH2CN 5(0,5)-4(0,4) |
| 100029.565 | SO N,J=5,4-4,4 |
| 100076.386 | HCCCN 11-10 |
| 100094.500 | CH2CO 5(1,5)-4(1,4) |
| 100109.744 | CH3CH213CN 11(1,10)-10(1,9) |
| 100110.270 | CH3SH 4(1)-3(1) A++ |
| 100122.000 | unidentified |
| 100155.839 | CH313CH2CN 11(1,10)-10(1,9) |
| 100157.000 | unidentified |
| 100173.100 | CH3SH 7(2)-8(1) A++ |
| 100185.000 | unidentified |
| 100197.200 | unidentified |
| 100200.400 | unidentified |
| 100240.584 | HCCCN 11-10 1v6 =1e |
Spectral window: 2
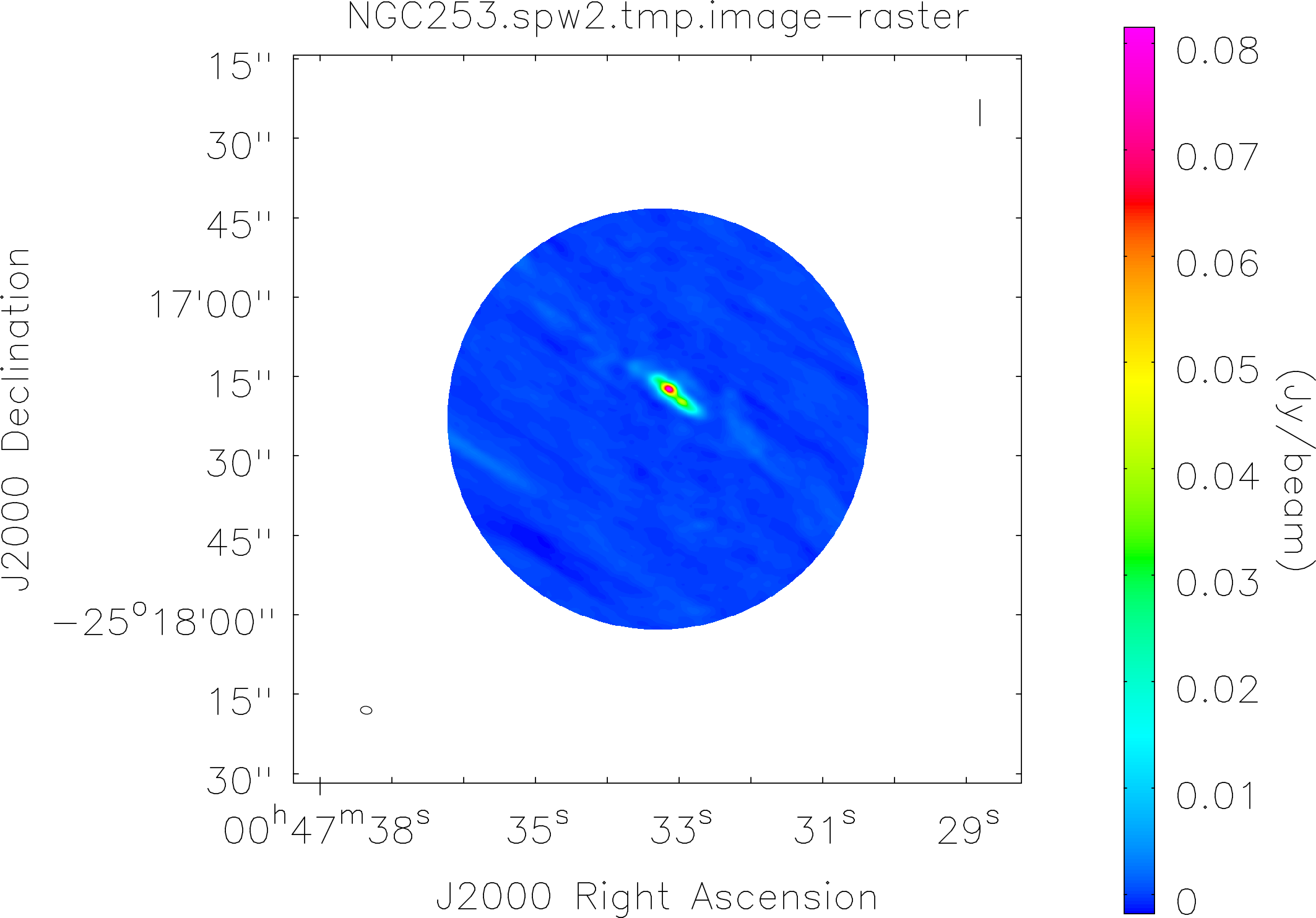
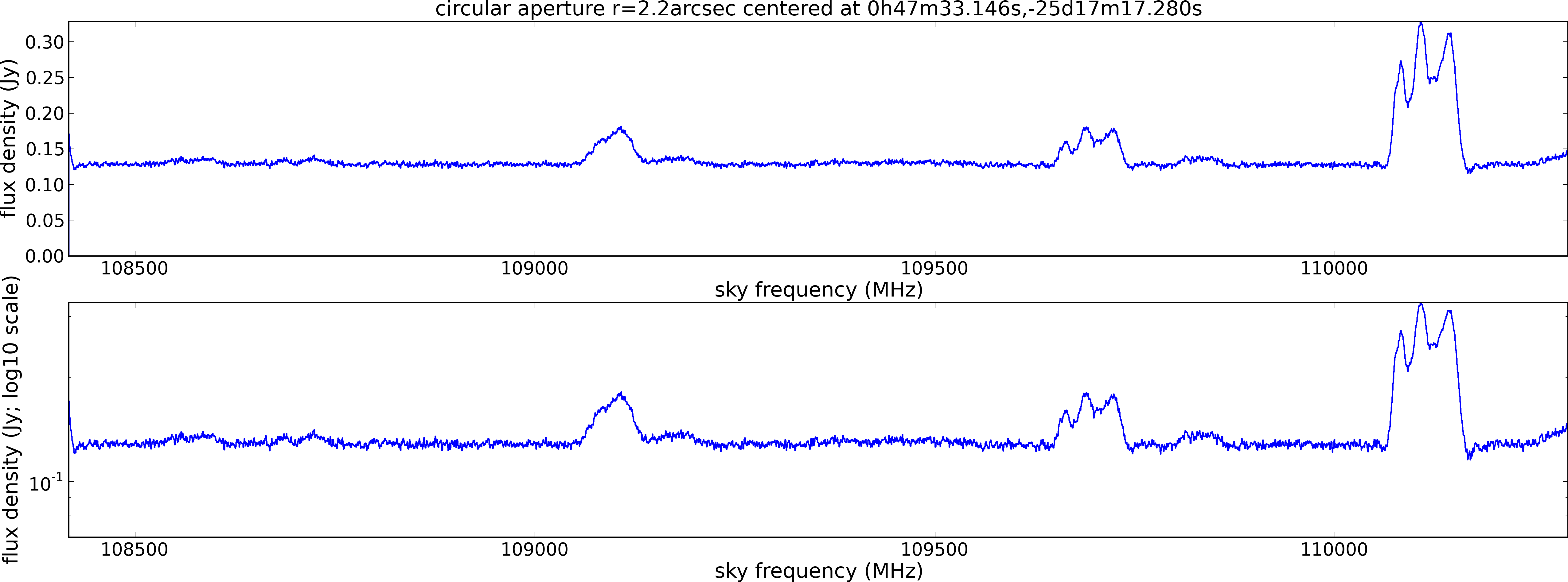
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 108426.900 | unidentified |
| 108434.511 | (CH3)2CO 14(2,12)-14(1,13) EE |
| 108438.616 | t-CH3CH2OH 13(3,10)-13(2,11) |
| 108444.200 | unidentified |
| 108453.000 | unidentified |
| 108471.967 | NaCN 7(0,7)-6(0,6) |
| 108484.474 | SiNC 21/2 J=17/2-15/2 f |
| 108507.700 | unidentified |
| 108510.601 | CH313CH2CN 12(2,10)-11(2,9) |
| 108511.200 | unidentified |
| 108514.400 | SiC2 5(1,5)-4(1,4) 1v3 |
| 108631.121 | 13CN F1=0,F2=1-0,F=0-1 |
| 108636.923 | 13CN F1=0,F2=1-0,F=1-1 |
| 108638.212 | 13CN F1=1,F2=1-1,F=1-0 |
| 108643.590 | 13CN F1=1,F2=1-1,F=2-1 |
| 108645.060 | 13CN F1=1,F2=1-1,F=0-1 |
| 108645.060 | 13CN F1=1,F2=1-1,F=1-1 |
| 108651.297 | 13CN J=1/2-1/2 F=2-1,F1=0,F2=1-0 |
| 108657.646 | 13CN J=1/2-1/2 F=2-2,F1=1,F2=1-1 |
| 108658.948 | 13CN J=1/2-1/2 F=1-2,F1=1,F2=1-1 |
| 108710.523 | HC13CCN 12-11 |
| 108721.008 | HCC13CN 12-11 |
| 108778.000 | unidentified |
| 108780.201 | 13CN J=3/2-1/2 F=3-2,F1=1,F2=2-1 |
| 108782.374 | 13CN J=3/2-1/2 F=2-1,F1=1,F2=2-1 |
| 108786.982 | 13CN J=3/2-1/2 F=1-0,F1=1,F2=2-1 |
| 108793.753 | 13CN J=3/2-1/2 F=1-1 |
| 108796.000 | unidentified |
| 108796.400 | 13CN J=3/2-1/2 F=2-2 |
| 108802.000 | unidentified |
| 108813.606 | CH2CHCN 20(1,19)-20(0,20) |
| 108834.270 | CCCN 11-10 J=23/2-21/2 |
| 108853.020 | CCCN 11-10 J=21/2-19/2 |
| 108866.000 | unidentified |
| 108883.548 | CH3OCHO 14(3,12)-14(2,13) A |
| 108893.929 | CH3OH 0(0,0)-1(-1,1) E |
| 108909.000 | unidentified |
| 108924.297 | SiS 6-5 |
| 108940.596 | CH3CH2CN 12(2,10)-11(2,9) |
| 108955.895 | SO2 39(6,34)-38(7,31) |
| 108987.000 | unidentified |
| 108998.000 | unidentified |
| 109008.670 | DCOOH 9(1,8)-9(0,9) |
| 109012.000 | unidentified |
| 109018.000 | unidentified |
| 109023.305 | HCCCN 12-11 1v4 |
| 109092.761 | CH2CHCHO 12(1,11)-11(1,10) |
| 109101.859 | CH3CH213CN 12(1,11)-11(1,10) |
| 109110.844 | O13CS 9-8 |
| 109125.744 | HC13CCN 12-11 1v7 =1f |
| 109137.570 | CH3OH 26(0,26)-26(-1,26) E |
| 109139.706 | HCC13CN 12-11 1v7 =1f - |
| 109153.210 | CH3OH 16(-2,15)-16(1,15) E |
| 109160.981 | HC5N 41-40 |
| 109164.048 | 13CH3OH 0(0,0)-1(-1,1) E |
| 109173.638 | HCCCN 12-11 |
| 109183.021 | HCCCN 12-11 1v5 =1e |
| 109244.222 | HCCCN 12-11 1v5 =1f |
| 109252.212 | SO N,J=3,2-2,1 |
| 109280.016 | CH2OHCHO 8(2,7)-7(1,6) |
| 109292.081 | CH3OCHO 10(1,9)-9(2,8) E |
| 109302.206 | CH3OCHO 10(1,9)-9(2,8) A |
| 109306.704 | HCCCN 12-11 1v4 1v7 =1e- |
| 109352.781 | HCCCN 12-11 1v6 =1e |
| 109378.534 | HC13CCN 12-11 2v7 =0 |
| 109382.031 | HC13CCN 12-11 2v7 =2e |
| 109386.800 | HC13CCN 12-11 2v7 =2f |
| 109438.720 | HCCCN 12-11 1v6 =1f |
| 109442.013 | HCCCN 12-11 1v7 =1e |
| 109463.063 | OCS 9-8 |
| 109469.409 | HCCCN 12-11 1v4 1v7 =1f+ |
| 109496.007 | HNCO 5(1,5)-4(1,4) |
| 109522.500 | HCCCN 12-11 2v6 =0 |
| 109530.000 | unidentified |
| 109538.000 | unidentified |
| 109549.500 | HCCCN 12-11 1v5 1v7 =0f+ |
| 109552.100 | HCCCN 12-11 1v5 1v7 =2f+ |
| 109558.000 | HCCCN 12-11 1v5 1v7 =0e- |
| 109563.700 | HCCCN 12-11 1v5 1v7 =2e- |
| 109571.390 | CH3OCH3 8(2,7)-8(1,8) EA |
| 109571.398 | CH3OCH3 8(2,7)-8(1,8) AE |
| 109574.119 | CH3OCH3 8(2,7)-8(1,8) EE |
| 109576.843 | CH3OCH3 8(2,7)-8(1,8) AA |
| 109598.818 | HCCCN 12-11 1v7 =1f |
| 109616.300 | HCCCN 12-11 2v6 =2 |
| 109650.301 | CH3CH2CN 12(1,11)-11(1,10) |
| 109689.610 | C15N 1-0 J=1/2-1/2 F=1-1 |
| 109720.000 | unidentified |
| 109738.500 | unidentified |
| 109753.499 | NH2CHO 5(1,4)-4(1,3) |
| 109757.587 | SO2 17(5,13)-18(4,14) |
| 109770.500 | unidentified |
| 109771.918 | HCCN N,J=5,6-4,5 |
| 109782.176 | C18O 1-0 |
| 109828.291 | CCCS 19-18 |
| 109833.489 | HNCO 5(3,2)-4(3,1) |
| 109833.489 | HNCO 5(3,3)-4(3,2) |
| 109861.999 | HCCN N,J=5,5-4,4 |
| 109862.778 | HCCCN 12-11 2v7 =0 |
| 109865.961 | HCCCN 12-11 2v7 =2e |
| 109870.278 | HNCO 5(1,5)-4(1,4) 6=1 |
| 109870.290 | HCCCN 12-11 2v7 =2f |
| 109872.366 | HNCO 5(2,4)-4(2,3) |
| 109872.773 | HNCO 5(2,3)-4(2,2) |
| 109905.753 | HNCO 5(0,5)-4(0,4) |
| 109943.300 | unidentified |
| 109943.453 | 13CH3CH2CN 13(1,13)-12(1,12) |
| 109959.600 | unidentified |
| 109972.000 | unidentified |
| 109990.000 | HCCCN 12-11 1v6 2v7 =1e- |
| 110023.540 | C15N 1-0 J=3/2-1/2 F=1-0 |
| 110024.590 | C15N 1-0 J=3/2-1/2 F=2-1 |
| 110035.600 | HCCCN 12-11 1v6 2v7 =-1f+ |
| 110036.200 | unidentified |
| 110046.249 | HCCN N,J=5,4-4,3 |
| 110050.804 | HCCCN 12-11 3v7 =1e |
| 110066.104 | HNCO 5(4 )-4(4 ) 5=1 |
| 110079.700 | unidentified |
| 110080.464 | HNCO 5(3 )-4(3 ) 6=1 |
| 110084.368 | HNCO 5(0,5)-4(0,4) 5=1 |
| 110084.727 | CH3CH2CN 4(2,3)-3(1,2) A t=1 |
| 110086.440 | HNCO 5(0,5)-4(0,4) 6=1 |
| 110089.279 | CH3CH2CN 4(2,2)-3(1,3) E t=1 |
| 110089.690 | HNCO 5(3 )-4(3 ) 5=1 |
| 110097.600 | HCCCN 12-11 1v6 2v7 =3 |
| 110098.000 | unidentified |
| 110104.112 | HNCO 5(2,4)-4(2,3) 5=1 |
| 110104.700 | unidentified |
| 110105.356 | HNCO 5(2,3)-4(2,2) 5=1 |
| 110105.400 | CH2DOH 9(1,8)-9(0,9) o1 |
| 110140.757 | C6H- 40-39 |
| 110143.300 | unidentified |
| 110148.800 | HCCCN 12-11 1v6 2v7 =-1e- |
| 110150.000 | unidentified |
| 110152.084 | NH2D 1(1,1)0- -1(0,1)0+ F=0-1 |
| 110152.995 | NH2D 1(1,1)0- -1(0,1)0+ F=2-1 |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ F=1-1 |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ F=2-2 |
| 110154.222 | NH2D 1(1,1)0- -1(0,1)0+ F=1-2 |
| 110155.053 | NH2D 1(1,1)0- -1(0,1)0+ F=1-0 |
| 110158.200 | unidentified |
| 110164.245 | HNCO 5(1,4)-4(1,3) 6=1 |
| 110188.860 | CH3OD 1(1,0)-1(0,1) E |
| 110189.800 | HCCCN 12-11 1v6 2v7 =1f+ |
| 110201.354 | 13CO 1-0 |
| 110211.796 | HCCCN 12-11 3v7 =3 |
| 110240.000 | unidentified |
| 110243.809 | C6H 21/2 J=79/2-77/2 f |
| 110262.640 | CH3OD 2(1,1)-2(0,2) E |
| 110275.978 | CH313CN 6(5)-5(5) |
Spectral window: 3
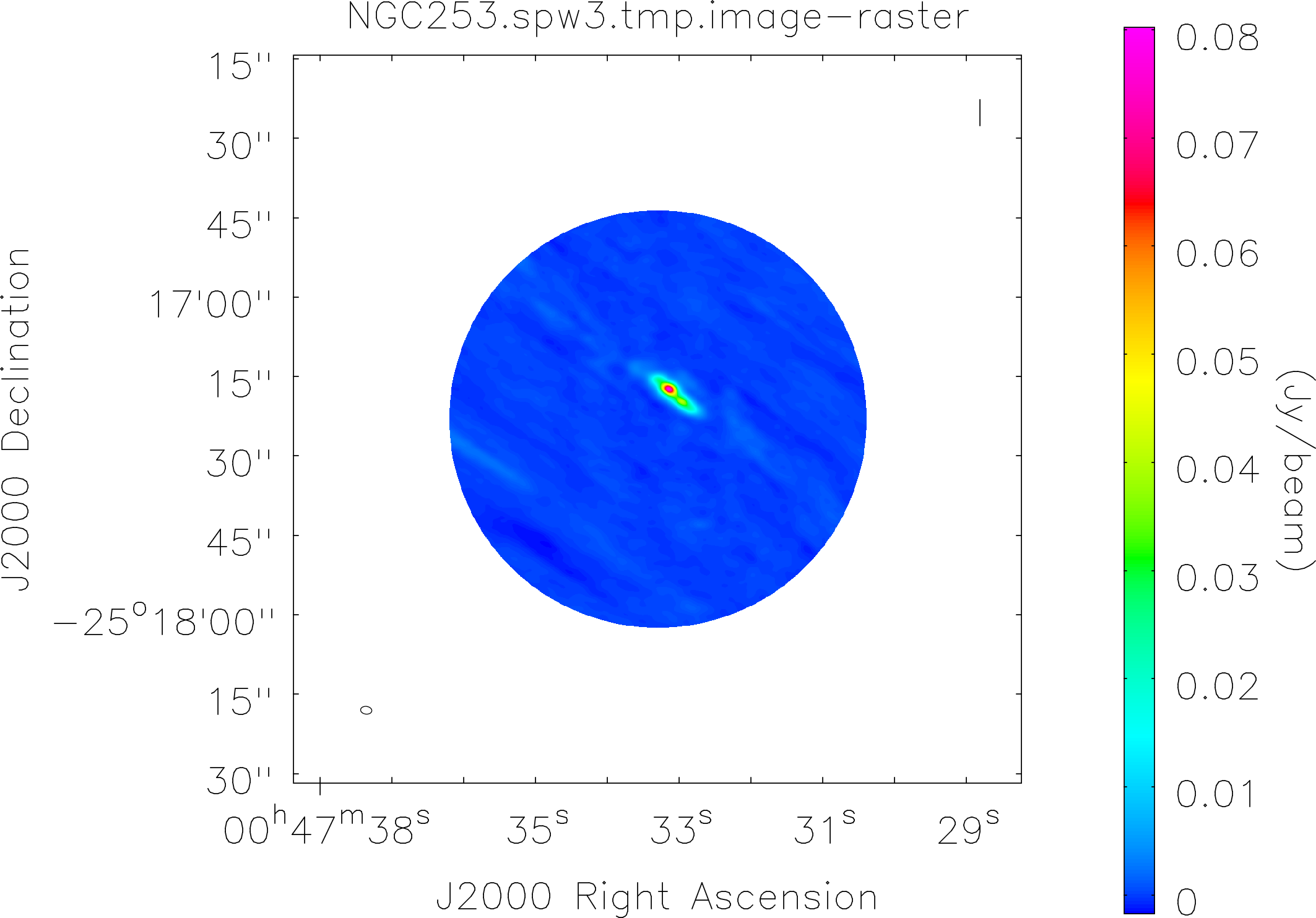
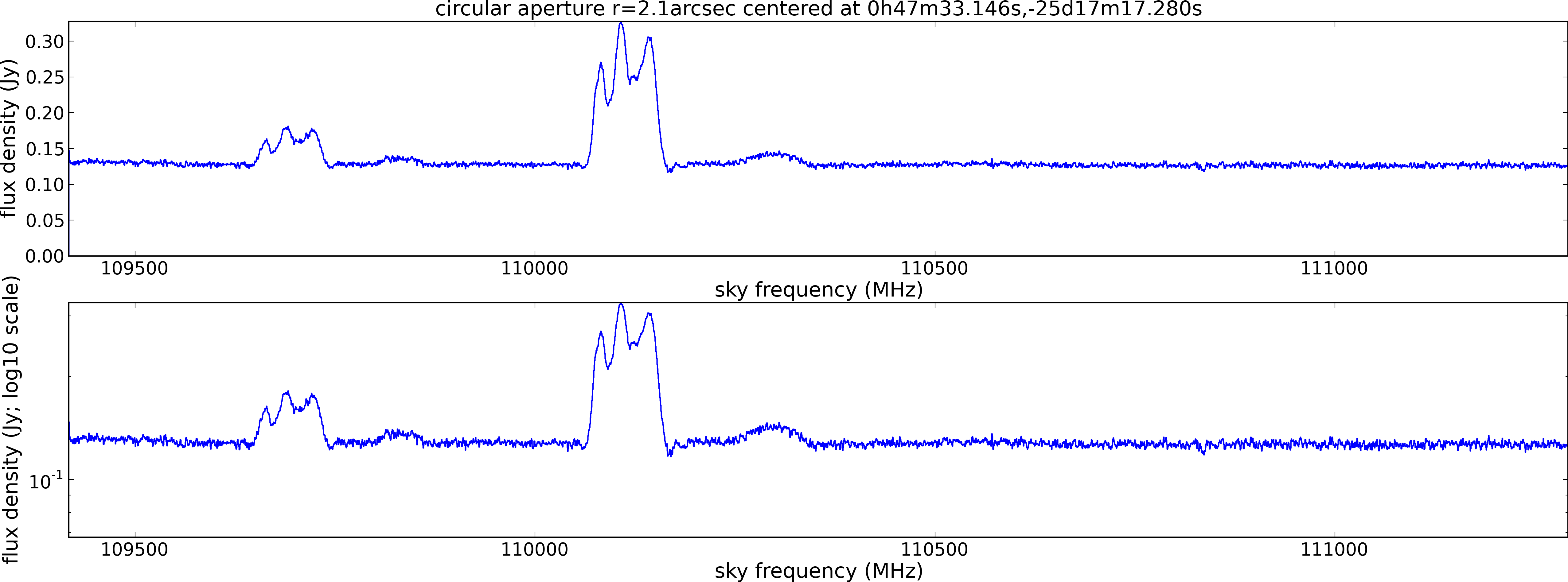
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 109438.720 | HCCCN 12-11 1v6 =1f |
| 109442.013 | HCCCN 12-11 1v7 =1e |
| 109463.063 | OCS 9-8 |
| 109469.409 | HCCCN 12-11 1v4 1v7 =1f+ |
| 109496.007 | HNCO 5(1,5)-4(1,4) |
| 109522.500 | HCCCN 12-11 2v6 =0 |
| 109530.000 | unidentified |
| 109538.000 | unidentified |
| 109549.500 | HCCCN 12-11 1v5 1v7 =0f+ |
| 109552.100 | HCCCN 12-11 1v5 1v7 =2f+ |
| 109558.000 | HCCCN 12-11 1v5 1v7 =0e- |
| 109563.700 | HCCCN 12-11 1v5 1v7 =2e- |
| 109571.390 | CH3OCH3 8(2,7)-8(1,8) EA |
| 109571.398 | CH3OCH3 8(2,7)-8(1,8) AE |
| 109574.119 | CH3OCH3 8(2,7)-8(1,8) EE |
| 109576.843 | CH3OCH3 8(2,7)-8(1,8) AA |
| 109598.818 | HCCCN 12-11 1v7 =1f |
| 109616.300 | HCCCN 12-11 2v6 =2 |
| 109650.301 | CH3CH2CN 12(1,11)-11(1,10) |
| 109689.610 | C15N 1-0 J=1/2-1/2 F=1-1 |
| 109720.000 | unidentified |
| 109738.500 | unidentified |
| 109753.499 | NH2CHO 5(1,4)-4(1,3) |
| 109757.587 | SO2 17(5,13)-18(4,14) |
| 109770.500 | unidentified |
| 109771.918 | HCCN N,J=5,6-4,5 |
| 109782.176 | C18O 1-0 |
| 109828.291 | CCCS 19-18 |
| 109833.489 | HNCO 5(3,2)-4(3,1) |
| 109833.489 | HNCO 5(3,3)-4(3,2) |
| 109861.999 | HCCN N,J=5,5-4,4 |
| 109862.778 | HCCCN 12-11 2v7 =0 |
| 109865.961 | HCCCN 12-11 2v7 =2e |
| 109870.278 | HNCO 5(1,5)-4(1,4) 6=1 |
| 109870.290 | HCCCN 12-11 2v7 =2f |
| 109872.366 | HNCO 5(2,4)-4(2,3) |
| 109872.773 | HNCO 5(2,3)-4(2,2) |
| 109905.753 | HNCO 5(0,5)-4(0,4) |
| 109943.300 | unidentified |
| 109943.453 | 13CH3CH2CN 13(1,13)-12(1,12) |
| 109959.600 | unidentified |
| 109972.000 | unidentified |
| 109990.000 | HCCCN 12-11 1v6 2v7 =1e- |
| 110023.540 | C15N 1-0 J=3/2-1/2 F=1-0 |
| 110024.590 | C15N 1-0 J=3/2-1/2 F=2-1 |
| 110035.600 | HCCCN 12-11 1v6 2v7 =-1f+ |
| 110036.200 | unidentified |
| 110046.249 | HCCN N,J=5,4-4,3 |
| 110050.804 | HCCCN 12-11 3v7 =1e |
| 110066.104 | HNCO 5(4 )-4(4 ) 5=1 |
| 110079.700 | unidentified |
| 110080.464 | HNCO 5(3 )-4(3 ) 6=1 |
| 110084.368 | HNCO 5(0,5)-4(0,4) 5=1 |
| 110084.727 | CH3CH2CN 4(2,3)-3(1,2) A t=1 |
| 110086.440 | HNCO 5(0,5)-4(0,4) 6=1 |
| 110089.279 | CH3CH2CN 4(2,2)-3(1,3) E t=1 |
| 110089.690 | HNCO 5(3 )-4(3 ) 5=1 |
| 110097.600 | HCCCN 12-11 1v6 2v7 =3 |
| 110098.000 | unidentified |
| 110104.112 | HNCO 5(2,4)-4(2,3) 5=1 |
| 110104.700 | unidentified |
| 110105.356 | HNCO 5(2,3)-4(2,2) 5=1 |
| 110105.400 | CH2DOH 9(1,8)-9(0,9) o1 |
| 110140.757 | C6H- 40-39 |
| 110143.300 | unidentified |
| 110148.800 | HCCCN 12-11 1v6 2v7 =-1e- |
| 110150.000 | unidentified |
| 110152.084 | NH2D 1(1,1)0- -1(0,1)0+ F=0-1 |
| 110152.995 | NH2D 1(1,1)0- -1(0,1)0+ F=2-1 |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ F=1-1 |
| 110153.599 | NH2D 1(1,1)0- -1(0,1)0+ F=2-2 |
| 110154.222 | NH2D 1(1,1)0- -1(0,1)0+ F=1-2 |
| 110155.053 | NH2D 1(1,1)0- -1(0,1)0+ F=1-0 |
| 110158.200 | unidentified |
| 110164.245 | HNCO 5(1,4)-4(1,3) 6=1 |
| 110188.860 | CH3OD 1(1,0)-1(0,1) E |
| 110189.800 | HCCCN 12-11 1v6 2v7 =1f+ |
| 110201.354 | 13CO 1-0 |
| 110211.796 | HCCCN 12-11 3v7 =3 |
| 110240.000 | unidentified |
| 110243.809 | C6H 21/2 J=79/2-77/2 f |
| 110262.640 | CH3OD 2(1,1)-2(0,2) E |
| 110275.978 | CH313CN 6(5)-5(5) |
| 110295.025 | CH313CN 6(4)-5(4) |
| 110298.098 | HNCO 5(1,4)-4(1,3) |
| 110299.514 | C6H 21/2 J=79/2-77/2 e |
| 110309.847 | CH313CN 6(3)-5(3) |
| 110320.438 | CH313CN 6(2)-5(2) |
| 110326.795 | CH313CN 6(1)-5(1) |
| 110328.914 | CH313CN 6(0)-5(0) |
| 110330.627 | CH3CN 6(5)-5(5) F=7-6 |
| 110330.872 | CH3CN 6(5)-5(5) F=5-4 |
| 110349.659 | CH3CN 6(4)-5(4) F=7-6 |
| 110349.797 | CH3CN 6(4)-5(4) F=5-4 |
| 110364.469 | CH3CN 6(3)-5(3) F=7-6 |
| 110364.524 | CH3CN 6(3)-5(3) F=5-4 |
| 110366.474 | HCCCN 12-11 3v7 =1f - |
| 110375.052 | CH3CN 6(2)-5(2) F=7-6 |
| 110381.404 | CH3CN 6(1)-5(1) F=7-6 |
| 110383.522 | CH3CN 6(0)-5(0) F=7-6 |
| 110413.590 | HCOOH 9(3,6)-10(2,9) |
| 110418.400 | unidentified |
| 110446.800 | unidentified |
| 110447.900 | unidentified |
| 110452.308 | t-CH3CH2OH 21(3,18)-21(2,19) |
| 110455.358 | CH3OCHO 9(8,1)-8(8,0) A |
| 110455.358 | CH3OCHO 9(8,2)-8(8,1) A |
| 110457.971 | CH3OCHO 9(8,2)-8(8,1) E |
| 110475.760 | CH3OD 3(1,2)-3(0,3) E |
| 110486.000 | unidentified |
| 110525.598 | CH3OCHO 9(7,2)-8(7,1) E |
| 110535.182 | CH3OCHO 9(7,3)-8(7,2) A |
| 110535.184 | CH3OCHO 9(7,2)-8(7,1) A |
| 110535.955 | CH3OCHO 9(7,3)-8(7,2) E |
| 110543.700 | HCCCN 12-11 4v7 =0 - |
| 110549.000 | HCCCN 12-11 4v7 =2f - |
| 110550.217 | CH3OCHO 7(2,6)-6(1,5) E |
| 110554.377 | HCCCN 12-11 4v7 =4 - |
| 110560.053 | CH3OCHO 7(2,6)-6(1,5) A |
| 110571.569 | CH3OCHO 10(0,10)-9(0,9) A t=1 |
| 110571.700 | unidentified |
| 110575.900 | unidentified |
| 110590.700 | unidentified |
| 110599.000 | unidentified |
| 110609.554 | CH3CN 6(1)-5(1) 8=1 =1 |
| 110637.370 | CH3CN 6(5)-5(5) 8=1 =-1 F=7-6 |
| 110642.900 | unidentified |
| 110652.678 | CH3OCHO 9(6,3)-8(6,2) E |
| 110660.869 | CH3CN 6(4)-5(4) 8=1 =-1 |
| 110661.057 | CH3CN 6(4)-5(4) 8=1 =-1 F=7-6 |
| 110662.261 | CH3OCHO 9(6,4)-8(6,3) E |
| 110663.263 | CH3OCHO 9(6,4)-8(6,3) A |
| 110663.456 | CH3OCHO 9(6,3)-8(6,2) A |
| 110675.500 | unidentified |
| 110680.350 | CH3CN 6(3)-5(3) 8=1 =-1 |
| 110683.959 | CH3CN 6(5)-5(5) 8=1 =1 |
| 110695.506 | CH3CN 6(2)-5(2) 8=1 =-1 1 |
| 110698.701 | CH3CN 6(4)-5(4) 8=1 =1 |
| 110706.251 | CH3CN 6(1)-5(1) 8=1 =-1 |
| 110709.313 | CH3CN 6(3)-5(3) 8=1 =+1 |
| 110712.166 | CH3CN 6(0)-5(0) 8=1 =1 1 |
| 110716.212 | CH3CN 6(2)-5(2) 8=1 =1 |
| 110732.510 | 13CH3OH 14(2,12)-13(1,13) A++ t=1 |
| 110756.092 | D2CS 4(1,4)-3(1,3) |
| 110765.000 | unidentified |
| 110770.500 | unidentified |
| 110776.400 | unidentified |
| 110776.488 | CH3OCHO 9(1,8)-8(1,7) A t=1 |
| 110788.590 | CH3OCHO 10(1,10)-9(1,9) E |
| 110790.533 | CH3OCHO 10(1,10)-9(1,9) A |
| 110812.590 | NHD2 1(1,0)-1(0,1) O- (a) |
| 110823.095 | CH3CN 6(1)-5(1) 8=1 =+-1 |
| 110837.821 | D2CO 2(1,2)-1(1,1) |
| 110839.975 | CH2CHCN 12(1,12)-11(1,11) |
| 110845.000 | unidentified |
| 110861.400 | unidentified |
| 110873.828 | CH3OCHO 9(5,4)-8(5,3) E |
| 110879.684 | CH3OCHO 9(3,7)-8(3,6) E |
| 110880.466 | CH3OCHO 9(5,5)-8(5,4) A |
| 110882.273 | CH3OCHO 9(5,5)-8(5,4) E |
| 110887.127 | CH3OCHO 9(3,7)-8(3,6) A |
| 110890.275 | CH3OCHO 9(5,4)-9(5,3) A |
| 110896.550 | NHD2 1(1,0)-1(0,1) O- (s) |
| 110900.900 | unidentified |
| 110906.000 | unidentified |
| 110912.900 | unidentified |
| 110918.765 | CH3OCHO 9(4,6)-8(4,4) E |
| 110924.900 | unidentified |
| 110931.103 | t-CH3CH2OH 15(8,8)-16(7,9) |
| 110931.153 | t-CH3CH2OH 15(8,7)-16(7,10) |
| 110938.300 | unidentified |
| 110950.750 | CH3OD 4(1,4)-4(0,4) E |
| 110954.500 | unidentified |
| 110962.074 | CH3OCHO 15(4,12)-15(3,13) A |
| 110968.000 | unidentified |
| 110977.300 | unidentified |
| 110986.400 | unidentified |
| 111006.600 | unidentified |
| 111013.400 | unidentified |
| 111021.800 | unidentified |
| 111029.100 | unidentified |
| 111034.600 | unidentified |
| 111038.000 | unidentified |
| 111047.500 | unidentified |
| 111069.000 | unidentified |
| 111094.105 | CH3OCHO 9(1,8)-8(1,7) E t=1 |
| 111094.900 | unidentified |
| 111121.400 | unidentified |
| 111127.900 | unidentified |
| 111139.000 | unidentified |
| 111161.900 | unidentified |
| 111169.831 | CH3OCHO 10(0,10)-9(0,9) E |
| 111171.659 | CH3OCHO 10(0,10)-9(0,9) A |
| 111195.990 | CH3OCHO 9(4,6)-8(4,5) A |
| 111211.000 | unidentified |
| 111223.397 | CH3OCHO 9(4,6)-8(4,5) E |
| 111243.339 | (CH3)2CO 10(1,9)-9(2,8) AE |
| 111243.388 | (CH3)2CO 10(2,9)-9(1,8) AE |
| 111243.424 | (CH3)2CO 10(1,9)-9(2,8) EA |
| 111243.472 | (CH3)2CO 10(2,9)-9(1,8) EA |
| 111254.400 | unidentified |
| 111262.067 | 13CH3CH2CN 13(0,13)-12(0,12) |
| 111267.540 | (CH3)2CO 10(*,9)-9(*,8) EE |
| 111289.601 | CH3OH 7(2,5)-8(1,8) A++ |
uv sampling
| FieldID | SpwID | uv_min(klambda) | uv_max(klambda) |
|---|---|---|---|
| 0 | 0 | 19.989 | 398.016 |
| 0 | 1 | 19.991 | 398.016 |
| 0 | 2 | 19.998 | 398.021 |
| 0 | 3 | 19.996 | 398.027 |
| 2 | 0 | 13.672 | 389.510 |
| 2 | 1 | 13.672 | 389.510 |
| 2 | 2 | 13.672 | 389.510 |
| 2 | 3 | 13.672 | 389.510 |
| 3 | 0 | 15.055 | 400.917 |
| 3 | 1 | 15.058 | 400.920 |
| 3 | 2 | 15.109 | 400.937 |
| 3 | 3 | 15.122 | 400.951 |
| 4 | 0 | 17.653 | 397.091 |
| 4 | 1 | 17.655 | 397.091 |
| 4 | 2 | 17.677 | 397.107 |
| 4 | 3 | 17.682 | 397.126 |
| 5 | 0 | 16.835 | 381.842 |
| 5 | 1 | 16.835 | 381.842 |
| 5 | 2 | 16.835 | 381.842 |
| 5 | 3 | 16.835 | 381.842 |