2011.0.00020.S/2011.0.00020.S_2011-12-26/2011.0.00020.S/sg_ouss_id/group_ouss_id/member_ouss_id/calibrated
full summary of this dataset can be found here
Spectral setup
| SpwID | Frame | #chan | skyfreq(MHz) | Chwid(kHz) | BW(MHz) | beam("x" deg) |
|---|---|---|---|---|---|---|
| 0 | TOPO | 3840 | 347985.5 ~ 349860.0 | 488.281 | 1875.000000 | 1.5 x 1.3, 76.5 |
| 1 | TOPO | 3840 | 349982.3 ~ 351856.8 | 488.281 | 1875.000000 | 1.4 x 1.3, 76.6 |
| 2 | TOPO | 3840 | 337743.8 ~ 339618.4 | 488.281 | 1875.000000 | 1.5 x 1.3, 77.8 |
| 3 | TOPO | 3840 | 336169.0 ~ 338043.6 | 488.281 | 1875.000000 | 1.5 x 1.3, 78.2 |
Observed fields
| FieldID | FieldName | R.A. | Dec | Epoch | nRows |
|---|---|---|---|---|---|
| 0 | 3c454.3 | 22:53:57.747936 | +16.08.53.56104 | J2000 | 4200 |
| 2 | Callisto | 02:04:22.204440 | +11.05.02.34941 | J2000 | 13500 |
| 3 | J0423-013 | 04:23:15.800736 | -01.20.33.06552 | J2000 | 12000 |
| 4 | NGC1614 | 04:34:00.026880 | -08.34.44.56920 | J2000 | 96000 |
Spectral window: 0
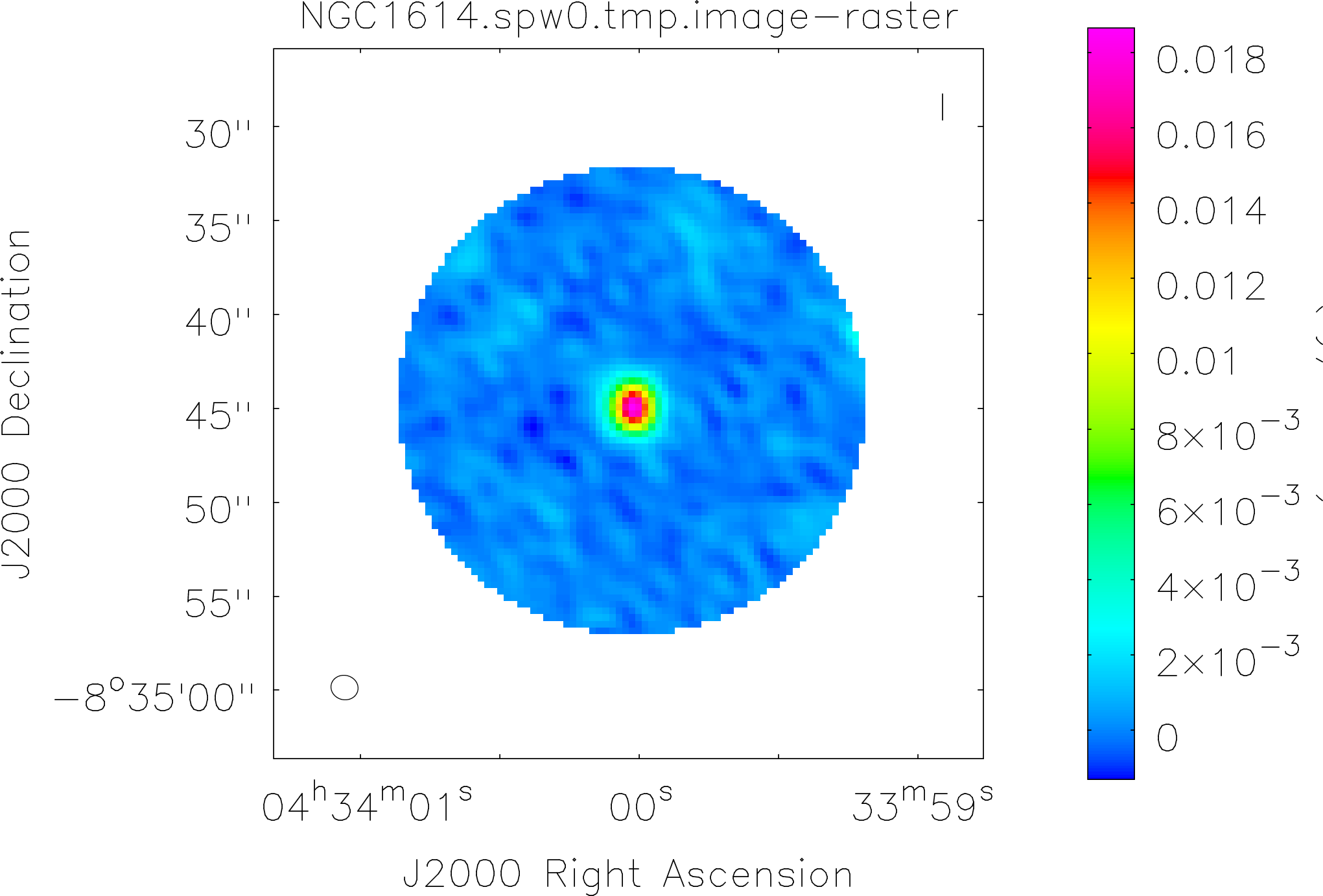
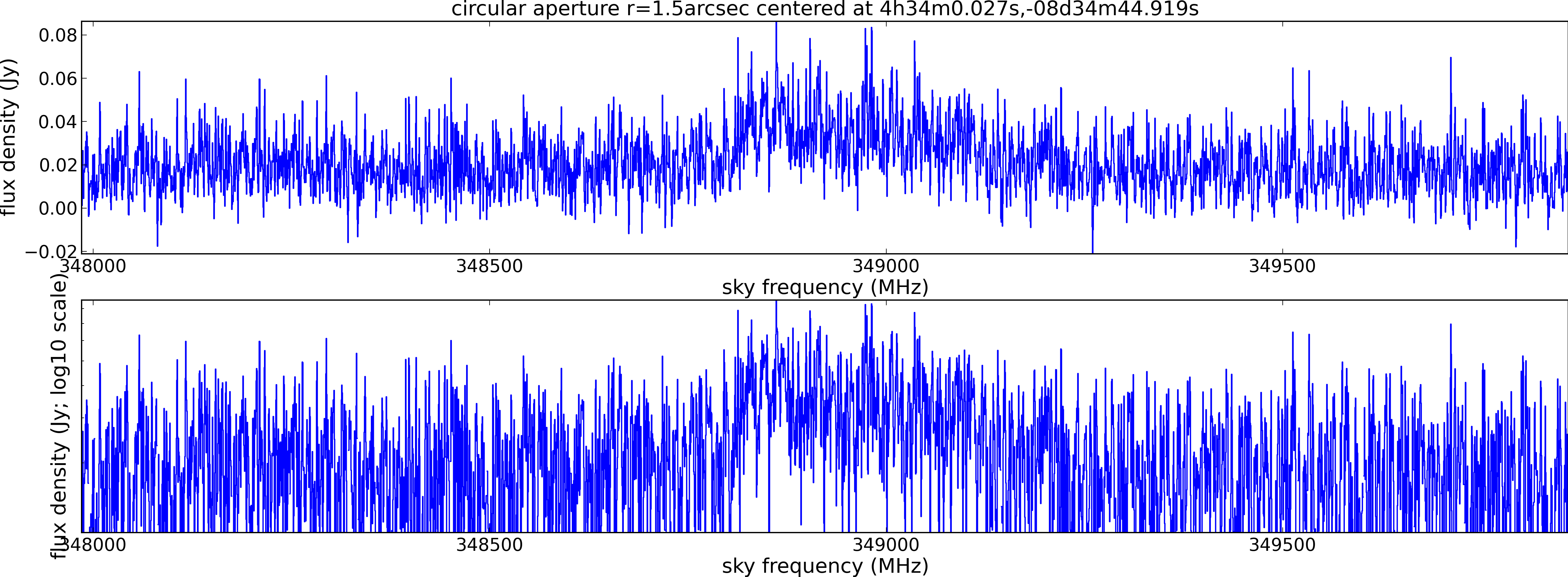
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 347991.839 | SO2 13(2,12)-12(1,11) 1v2 |
| 348100.194 | 13CH3OH 11(0,11)-10(1,9) E |
| 348117.481 | 34SO2 19(4,16)-19(3,17) |
| 348202.600 | unidentified |
| 348262.000 | HCOOD 16(8,8)-15(8,7) |
| 348269.000 | unidentified |
| 348330.500 | unidentified |
| 348340.490 | HN13C 4-3 |
| 348387.835 | SO2 24(2,22)-23(3,21) |
| 348518.390 | HNO 1(1,1)-2(0,2) |
| 348534.240 | H2CS 10(1,9)-9(1,8) |
| 348637.060 | CH2CHCN 38(1,38)-37(0,37) |
| 348909.527 | CH3OCHO 28(9,20)-27(9,19) E |
| 348911.425 | CH3CN 19(9)-18(9) |
| 348915.019 | CH3OCHO 28(9,20)-27(9,19) A |
| 349024.989 | CH3CN 19(8)-18(8) |
| 349106.954 | CH3OH 14(1,13)-14(0,14) A-+ |
| 349125.301 | CH3CN 19(7)-18(7) |
| 349212.320 | CH3CN 19(6)-18(6) |
| 349286.012 | CH3CN 19(5)-18(5) |
| 349337.741 | C2H 4-3 J=9/2-7/2 F=5-4 |
| 349339.067 | C2H 4-3 J=9/2-7/2 F=4-3 |
| 349346.346 | CH3CN 19(4)-18(4) |
| 349393.298 | CH3CN 19(3)-18(3) |
| 349399.342 | C2H 4-3 J=7/2-5/2 F=4-3 |
| 349400.692 | C2H 4-3 J=7/2-5/2 F=3-2 |
| 349426.849 | CH3CN 19(2)-18(2) |
| 349446.985 | CH3CN 19(1)-18(1) |
| 349453.698 | CH3CN 19(0)-18(0) |
| 349783.325 | SO2 46(5,41)-46(4,42) |
| 349802.991 | CH3OCH3 10(1,10)-11(2,9) EA |
| 349802.993 | CH3OCH3 10(1,10)-11(2,9) AE |
| 349806.165 | CH3OCH3 10(1,10)-11(2,9) EE |
| 349809.338 | CH3OCH3 10(1,10)-11(2,9) AA |
Spectral window: 1
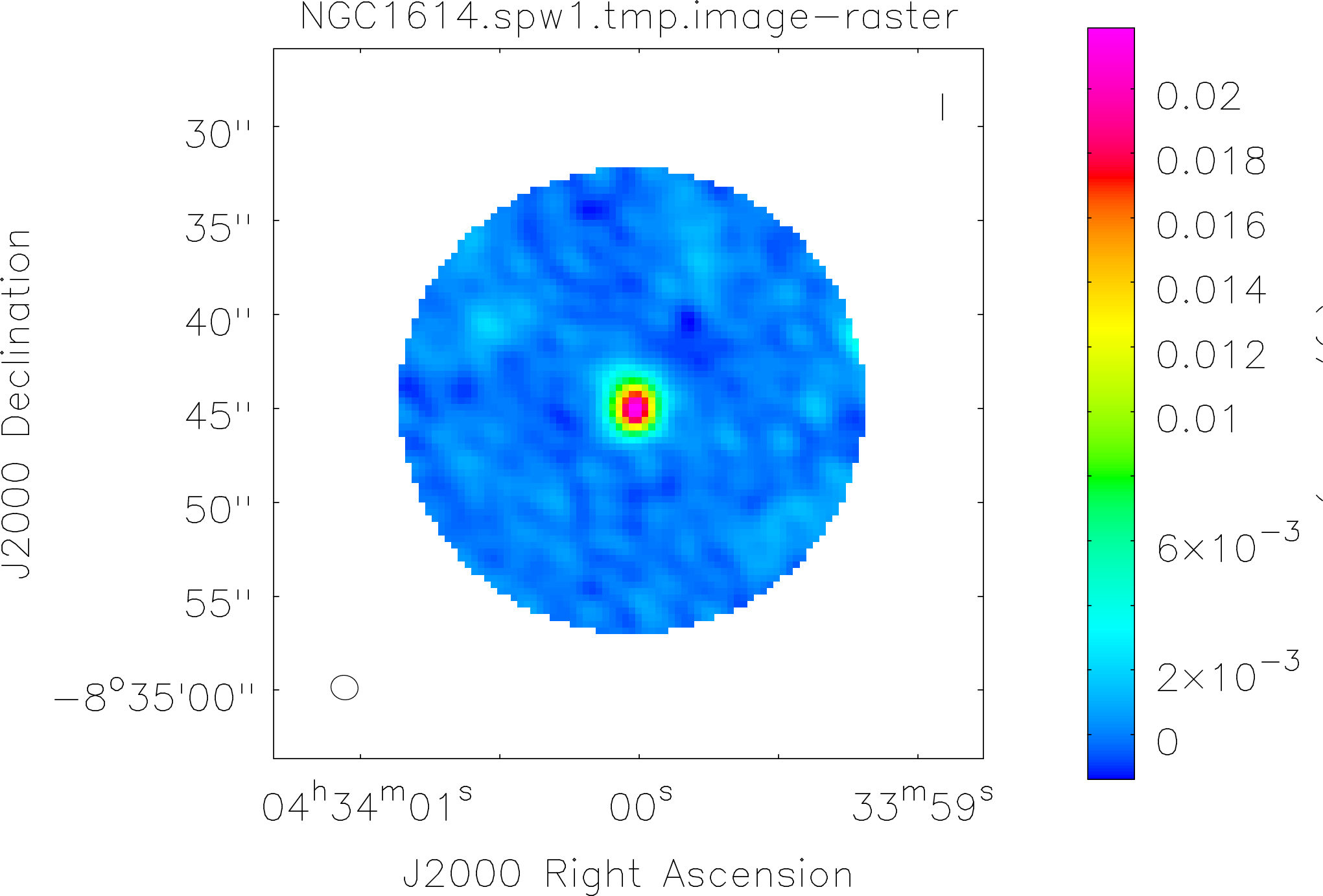
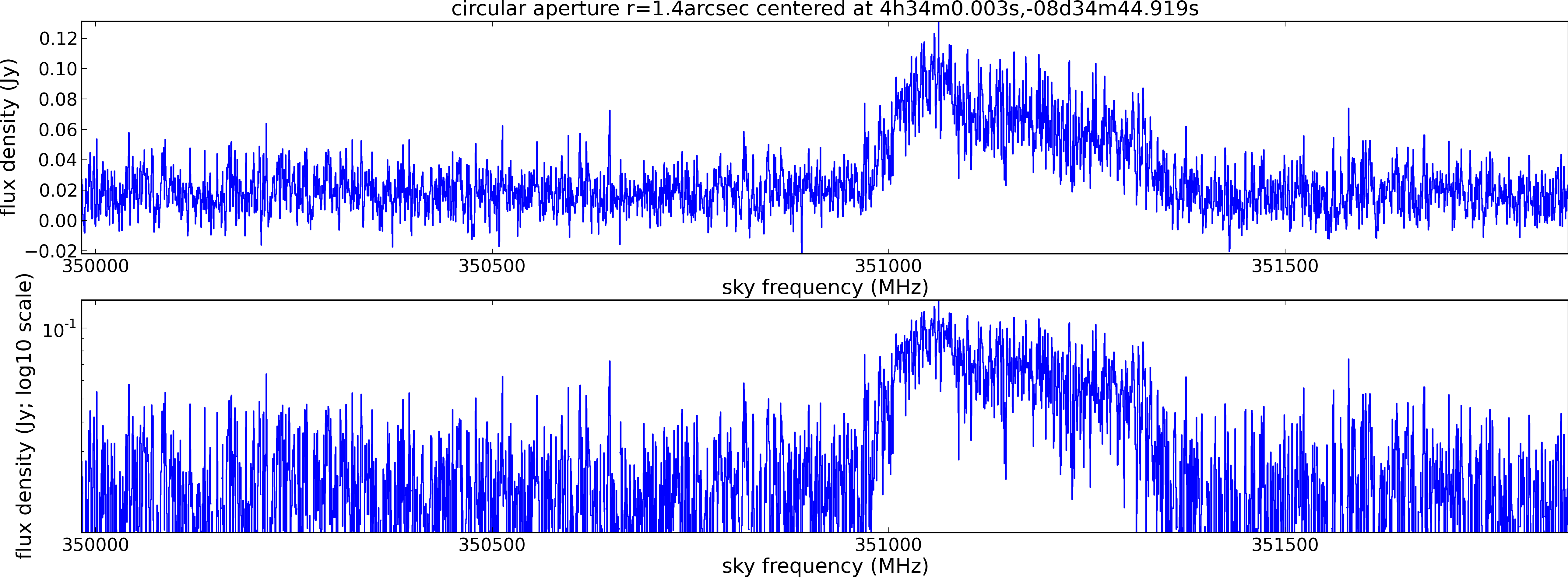
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 349995.400 | unidentified |
| 350035.652 | 30SiS 20-19 |
| 350103.084 | 13CH3OH 1(1,1)-0(0,0) A++ |
| 350149.000 | unidentified |
| 350169.900 | unidentified |
| 350280.000 | SiC2 15(10,*)-14(10,*) |
| 350286.900 | unidentified |
| 350333.340 | HNCO 16(1,16)-15(1,15) |
| 350423.500 | CH3CN 18(-2)-17(-2) 8=1 =+1 |
| 350423.500 | CH3CN 18(2)-17(2) 8=1 =-1 |
| 350449.530 | CH3CN 18(-1)-17(-1) 8=1 =+1 |
| 350515.000 | unidentified |
| 350552.230 | CH3CN 18(2)-17(2) 8=1 =+1 |
| 350687.651 | CH3OH 4(0,4)-3(-1,3) E |
| 350804.400 | unidentified |
| 350847.100 | unidentified |
| 350862.718 | SO2 10(6,4)-11(5,7) |
| 350905.070 | CH3OH 1(1,1)-0(0,0) A++ |
| 351015.853 | CH3OCHO 28(7,22)-27(7,21) A |
| 351043.525 | NO J,F=7/2,9/2-5/2,7/2 f |
| 351047.000 | unidentified |
| 351051.524 | NO J,F=7/2,7/2-5/2,5/2 f |
| 351051.798 | NO J,F=7/2,5/2-5/2,3/2 f |
| 351236.343 | CH3OH 9(5,5)-10(4,6) E |
| 351257.205 | SO2 5(3,3)-4(2,2) |
| 351289.990 | SO2 36(5,31)-36(4,32) 1v2 |
| 351420.100 | unidentified |
| 351454.240 | CH2NH 10(1,9)-10(0,10) |
| 351539.400 | unidentified |
| 351553.900 | unidentified |
| 351633.400 | HNCO 16(0,16)-15(0,15) |
| 351768.648 | H2CO 5(1,5)-4(1,4) |
| 351822.700 | unidentified |
Spectral window: 2
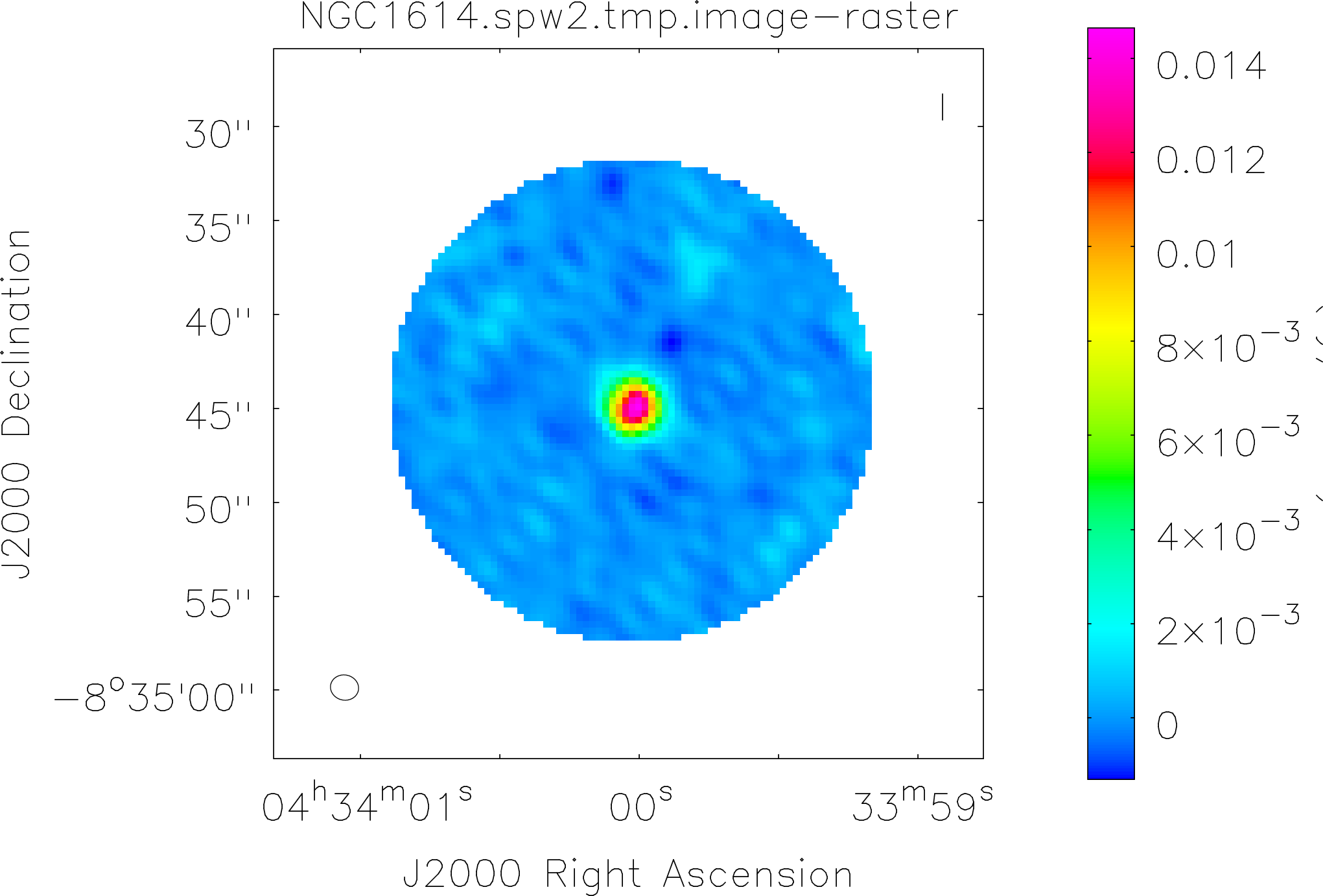
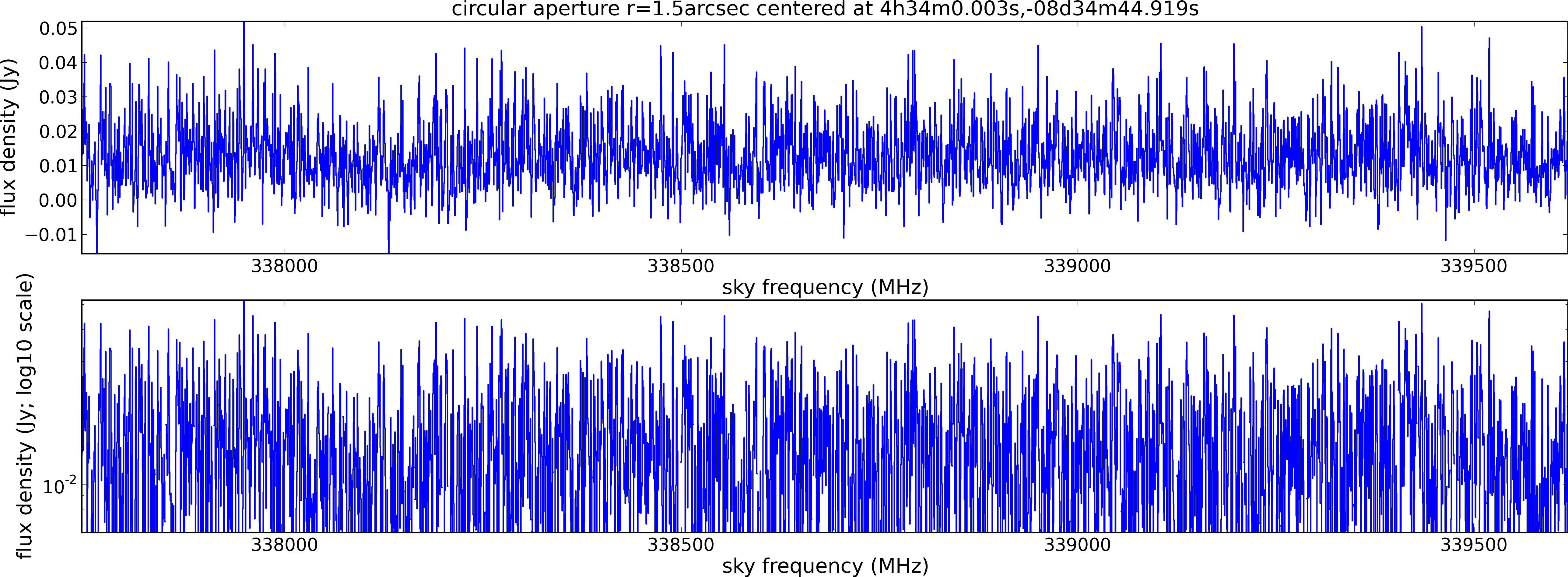
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 337748.771 | CH3OH 7(0,7)-6(0,6) A++ t=1 |
| 337770.614 | CH3OCH3 7(4,4)-6(3,4) EA |
| 337778.025 | CH3OCH3 7(4,4)-6(3,4) EE |
| 337779.470 | CH3OCH3 7(4,3)-6(3,4) AE |
| 337785.100 | HCOOH 15(5,11)-14(5,10) |
| 337787.216 | CH3OCH3 7(4,3)-6(3,4) AA |
| 337787.800 | HCOOH 15(5,10)-14(5,9) |
| 337787.863 | CH3OCH3 7(4,3)-6(3,4) EE |
| 337790.076 | CH3OCH3 7(4,3)-6(3,4) EA |
| 337811.639 | CH3OCHO 44(6,39)-44(5,40) A |
| 337825.275 | HCCCN 37-36 1v7 =1f |
| 337829.100 | unidentified |
| 337839.300 | unidentified |
| 337844.400 | unidentified |
| 337877.500 | CH3OH 7(1,6)-6(1,5) A t=2 |
| 337892.524 | SO2 21(2,20)-21(1,21) 1v2 |
| 337938.089 | CH3OH 20(-6,14)-21(-5,16) E |
| 337969.434 | CH3OH 7(1,6)-6(1,5) A-- t=1 |
| 337969.600 | unidentified |
| 337973.000 | unidentified |
| 338083.190 | H2CS 10(1,10)-9(1,9) |
| 338099.107 | t-CH3CH2OH 18(7,11)-18(6,12) |
| 338109.733 | t-CH3CH2OH 18(7,12)-18(6,13) |
| 338124.498 | CH3OH 7(0,7)-6(0,6) E |
| 338142.834 | CH3CH2CN 37(3,34)-36(3,33) |
| 338143.700 | HCOOH 15(4,12)-14(4,11) |
| 338147.000 | unidentified |
| 338201.800 | HCOOH 15(3,13)-14(3,12) |
| 338204.003 | c-C3H2 5(5,1)-4(4,0) |
| 338213.506 | CH2CHCN 37(1,37)-36(1,36) |
| 338248.700 | HCOOH 15(4,11)-14(4,10) |
| 338278.149 | CH2CHCN 35(2,33)-34(2,32) |
| 338305.976 | SO2 18(4,14)-18(3,15) |
| 338320.348 | 34SO2 13(2,12)-12(1,11) |
| 338338.014 | CH3OCHO 27(8,19)-26(8,18) E |
| 338344.605 | CH3OH 7(-1,7)-6(-1,6) E |
| 338355.823 | CH3OCHO 27(8,19)-26(8,18) A |
| 338396.331 | CH3OCHO 27(7,21)-26(7,20) E |
| 338404.593 | CH3OH 7(6,2)-6(6,1) E |
| 338408.718 | CH3OH 7(0,7)-6(0,6) A++ |
| 338414.117 | CH3OCHO 27(7,21)-26(7,20) A |
| 338430.981 | CH3OH 7(-6,1)-6(-6,0) E |
| 338442.367 | CH3OH 7(6,1)-6(6,0) A++ |
| 338442.367 | CH3OH 7(6,2)-6(6,1) A-- |
| 338447.260 | 29SiS 19-18 |
| 338447.691 | CH2CHCN 37(0,37)-36(0,36) |
| 338456.521 | CH3OH 7(-5,2)-6(-5,1) E |
| 338475.217 | CH3OH 7(5,3)-6(5,2) E |
| 338486.322 | CH3OH 7(5,2)-6(5,1) A-- |
| 338486.322 | CH3OH 7(5,3)-6(5,2) A++ |
| 338504.056 | CH3OH 7(-4,4)-6(-4,3) E |
| 338512.627 | CH3OH 7(4,4)-6(4,3) A-- |
| 338512.639 | CH3OH 7(4,3)-6(4,2) A++ |
| 338512.856 | CH3OH 7(2,6)-6(2,5) A-- |
| 338530.256 | CH3OH 7(4,3)-6(4,2) E |
| 338540.824 | CH3OH 7(3,5)-6(3,4) A++ |
| 338543.149 | CH3OH 7(3,4)-6(3,3) A-- |
| 338559.963 | CH3OH 7(-3,5)-6(-3,4) E |
| 338583.223 | CH3OH 7(3,4)-6(3,3) E |
| 338611.816 | SO2 20(4,19)-19(2,18) |
| 338614.953 | CH3OH 7(1,6)-6(1,5) E |
| 338639.807 | CH3OH 7(2,5)-6(2,4) A++ |
| 338708.800 | unidentified |
| 338721.694 | CH3OH 7(2,5)-6(2,4) E |
| 338722.914 | CH3OH 7(-2,6)-6(-2,5) E |
| 338747.000 | unidentified |
| 338759.800 | unidentified |
| 338759.948 | 13CH3OH 13(0,13)-12(1,12) A++ |
| 338771.400 | unidentified |
| 338773.000 | unidentified |
| 338785.692 | 34SO2 14(4,10)-14(3,11) |
| 338788.813 | CH3CH2CN 39(1,38)-38(2,37) |
| 338821.000 | unidentified |
| 338843.400 | unidentified |
| 338886.171 | t-CH3CH2OH 15(7,8)-15(6,9) |
| 338887.356 | t-CH3CH2OH 15(7,9)-15(6,10) |
| 338929.360 | 30SiO 8-7 v=0 |
| 339061.026 | t-CH3CH2OH 14(7,7)-14(6,8) |
| 339061.537 | t-CH3CH2OH 14(7,8)-14(6,9) |
| 339129.374 | CH3OCHO 13(7,7)-12(6,7) E |
| 339137.100 | unidentified |
| 339149.160 | SO2 37(4,34)-38(1,37) |
| 339152.730 | CH3OCHO 13(7,6)-12(6,6) E |
| 339185.942 | CH3OCHO 13(7,7)-12(6,7) A |
| 339196.327 | CH3OCHO 13(7,6)-12(6,6) A |
| 339201.539 | t-CH3CH2OH 13(7,6)-13(6,7) |
| 339201.745 | t-CH3CH2OH 13(7,7)-13(6,8) |
| 339309.002 | 13CH3CN 19(3)-18(3) |
| 339312.559 | t-CH3CH2OH 12(7,5)-12(6,6) |
| 339312.635 | t-CH3CH2OH 12(7,6)-12(6,7) |
| 339340.824 | 13CH3CN 19(2)-18(2) |
| 339341.502 | SO N,J=3,3-2,3 |
| 339353.782 | O13CS 28-27 |
| 339359.923 | 13CH3CN 19(1)-18(1) |
| 339366.290 | 13CH3CN 19(0)-18(0) |
| 339398.498 | t-CH3CH2OH 11(7,4)-11(6,5) |
| 339398.524 | t-CH3CH2OH 11(7,5)-11(6,6) |
| 339446.779 | CN 3-2 J=5/2-5/2 F=3/2-3/2 |
| 339459.994 | CN 3-2 J=5/2-5/2 F=3/2-5/2 |
| 339462.679 | CN 3-2 J=5/2-5/2 F=5/2-3/2 |
| 339463.379 | t-CH3CH2OH 10(7,3)-10(6,4) |
| 339463.387 | t-CH3CH2OH 10(7,4)-10(6,5) |
| 339475.894 | CN 3-2 J=5/2-5/2 F=5/2-5/2 |
| 339491.485 | CH3OCH3 19(1,18)-18(2,17) AA |
| 339491.549 | CH3OCH3 19(1,18)-18(2,17) EE |
| 339491.613 | CH3OCH3 19(1,18)-18(2,17) AE+EA |
| 339493.281 | CN 3-2 J=5/2-5/2 F=5/2-7/2 |
| 339499.303 | CN 3-2 J=5/2-5/2 F=7/2-5/2 |
| 339510.854 | t-CH3CH2OH 9(7,2)-9(6,3) |
| 339510.856 | t-CH3CH2OH 9(7,3)-9(6,4) |
| 339516.690 | CN 3-2 J=5/2-5/2 F=7/2-7/2 |
| 339544.224 | t-CH3CH2OH 8(7,1)-8(6,2) |
| 339544.225 | t-CH3CH2OH 8(7,2)-8(6,3) |
| 339566.451 | t-CH3CH2OH 7(7,*)-7(6,*) |
Spectral window: 3
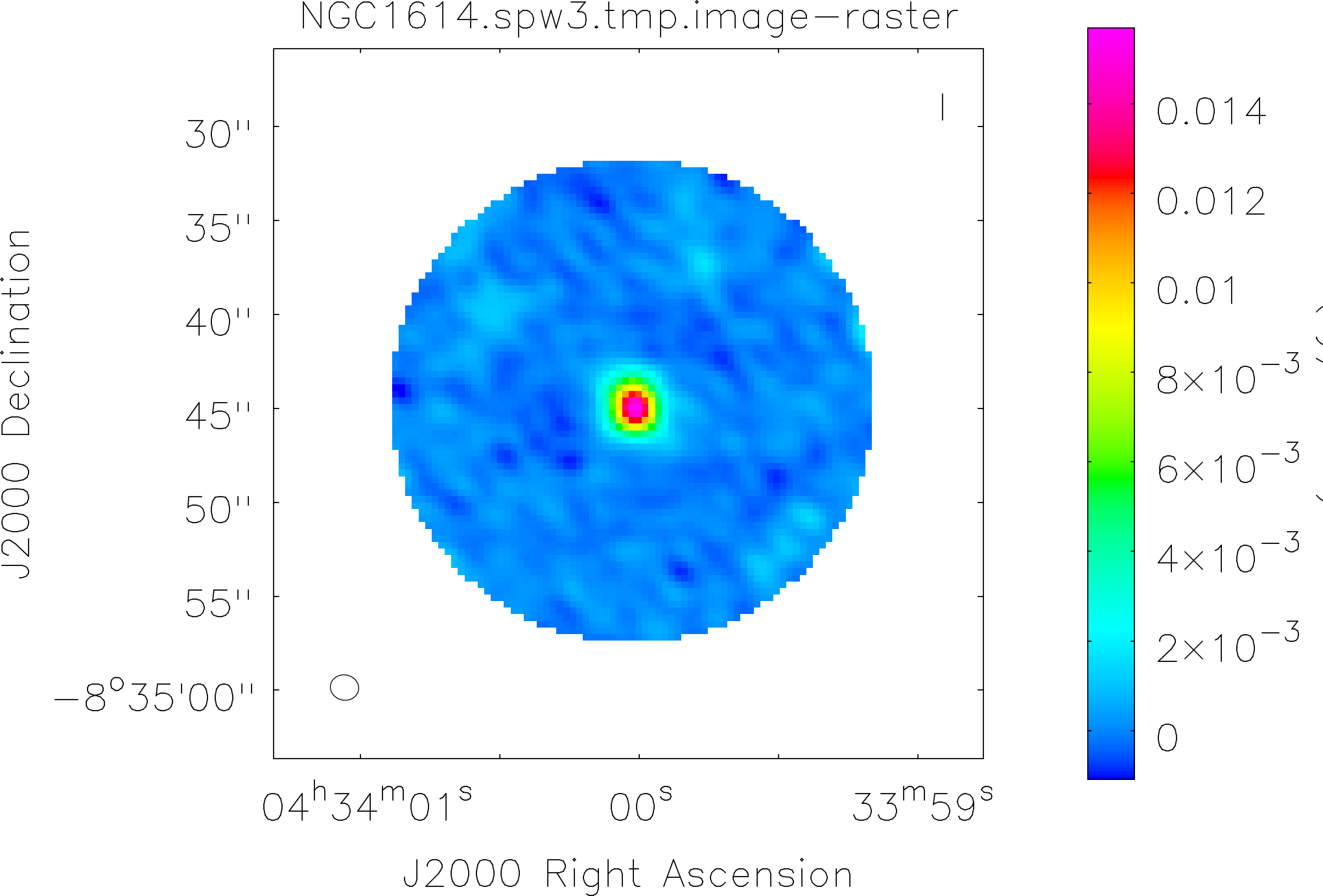
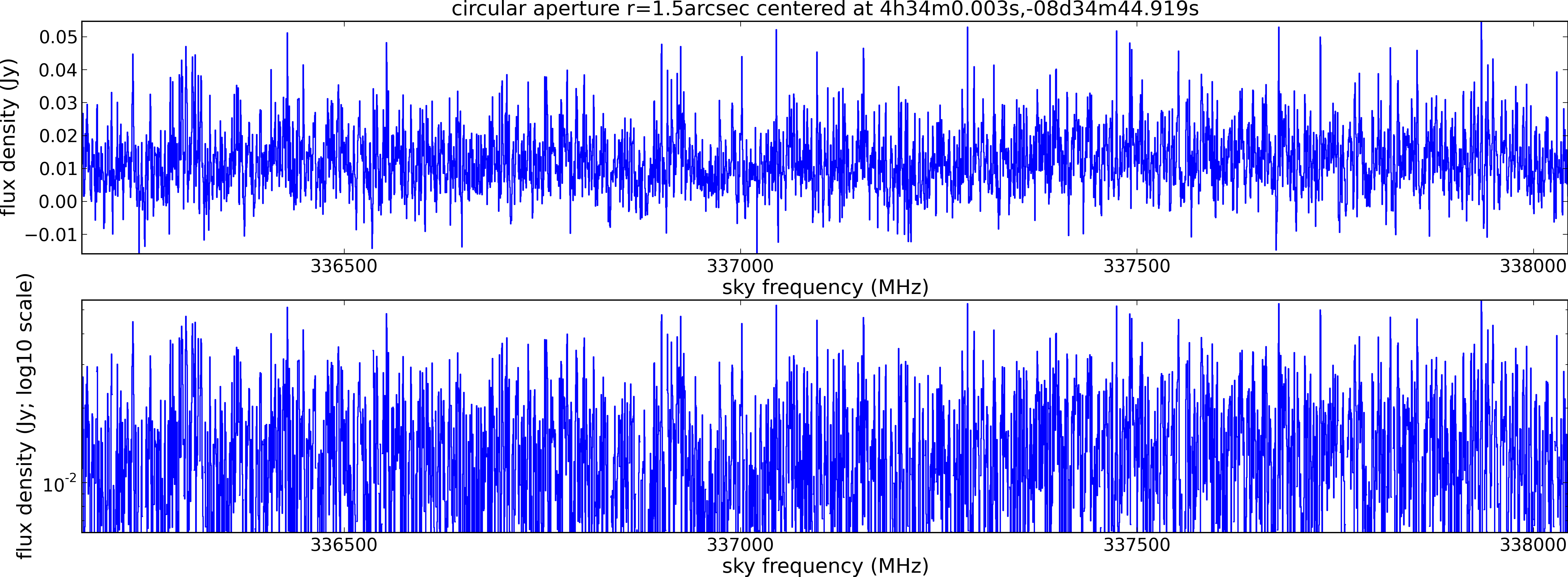
Lovas recommendation (http://physics.nist.gov/cgi-bin/micro/table5/start.pl)
click table head to collapse/expand
| Rest frequency (MHz) | Molecular transition |
|---|---|
| 336351.347 | CH3OCHO 27(6,22)-26(6,21) E |
| 336352.205 | NH2CHO 29(4,25)-29(3,26) |
| 336354.806 | CH3OCHO 26(5,21)-25(5,20) E |
| 336368.188 | CH3OCHO 27(6,22)-26(6,21) A |
| 336373.824 | CH3OCHO 26(5,21)-25(5,20) A |
| 336438.219 | CH3OH 14(7,7)-15(6,10) A-- |
| 336438.219 | CH3OH 14(7,8)-15(6,9) A++ |
| 336520.080 | HCCCN 37-36 |
| 336553.750 | SO N,J=11,10-10,10 |
| 336605.800 | CH3OH 7(1,7)-6(1,6) A++ t=2 |
| 336614.038 | CH3CH2CN 20(4,17)-19(3,16) |
| 336638.000 | unidentified |
| 336669.515 | SO2 16(7,9)-17(6,12) |
| 336760.698 | SO2 20(1,19)-19(2,18) 1v2 |
| 336808.458 | CH2CHCN 37(0,37)-36(1,36) |
| 336865.153 | CH3OH 12(1,11)-12(0,12) A-+ |
| 336887.200 | unidentified |
| 336889.203 | CH3OCHO 26(6,20)-25(6,19) E |
| 336918.151 | CH3OCHO 26(6,20)-25(6,19) A |
| 337029.500 | CH3OH 7(2,5)-6(2,4) A++ t=2 |
| 337029.500 | CH3OH 7(2,6)-6(2,5) A-- t=2 |
| 337039.740 | CH2CHCN 36(2,35)-35(2,34) |
| 337050.914 | CH2CHCN 35(3,32)-34(3,31) |
| 337061.104 | C17O 3-2 |
| 337098.500 | CH3OH 7(5,2)-6(5,1) A-- t=2 |
| 337098.500 | CH3OH 7(5,3)-6(5,2) A++ t=2 |
| 337113.800 | CH3OH 7(1,7)-6(1,6) E t=2 |
| 337135.858 | CH3OH 3(3,0)-4(2,2) E |
| 337149.800 | unidentified |
| 337159.400 | CH3OH 7(6,1)-6(6,0) E t=2 |
| 337167.000 | unidentified |
| 337175.200 | CH3OH 7(-4,3)-6(-4,2) E t=2 |
| 337186.600 | CH3OH 7(0,7)-6(0,6) E t=2 |
| 337198.400 | CH3OH 7(-5,3)-6(-5,2) E t=2 |
| 337199.730 | 33SO N,J=8,7-7,6 |
| 337201.000 | unidentified |
| 337252.300 | CH3OH 7(3,4)-6(3,3) A-- t=2 |
| 337252.300 | CH3OH 7(3,5)-6(3,4) A++ t=2 |
| 337273.600 | CH3OH 7(4,3)-6(4,2) A++ t=2 |
| 337273.600 | CH3OH 7(4,4)-6(4,3) A-- t=2 |
| 337278.900 | CH3OH 7(-2,5)-6(-2,4) E t=2 |
| 337284.400 | CH3OH 7(0,7)-6(0,6) A t=2 |
| 337296.000 | CH3OH 7(3,5)-6(3,4) E t=2 |
| 337297.483 | CH3OH 7(1,7)-6(1,6) A++ t=1 |
| 337300.924 | NH2CHO 19(2,18)-19(1,19) |
| 337302.900 | CH3OH 7(2,6)-6(2,5) E t=2 |
| 337312.300 | CH3OH 7(-1,6)-6(-1,5) E t=2 |
| 337323.531 | t-CH3CH2OH 20(7,14)-20(6,15) |
| 337344.692 | HCCCN 37-36 1v7 =1e |
| 337347.559 | CH3CH2CN 38(3,36)-37(3,35) |
| 337353.000 | unidentified |
| 337396.459 | C34S 7-6 |
| 337420.484 | CH3OCH3 21(2,19)-20(3,18) AA |
| 337421.032 | CH3OCH3 21(2,19)-20(3,18) EE |
| 337421.580 | CH3OCH3 21(2,19)-20(3,18) AE+EA |
| 337427.800 | NH2CN 17(1,17)-16(1,16) v=1 |
| 337445.858 | CH3CH2CN 37(4,33)-36(4,32) |
| 337461.600 | unidentified |
| 337463.624 | CH3OH 7(6,1)-6(6,0) A++ t=1 |
| 337473.100 | unidentified |
| 337489.669 | CH3OCHO 27(8,20)-26(8,19) E |
| 337490.378 | CH3OH 7(-6,2)-6(-6,1) E t=1 |
| 337490.500 | HCOOH 15(7,*)-14(7,*) |
| 337503.489 | CH3OCHO 27(8,20)-26(8,19) A |
| 337519.117 | CH3OH 7(3,5)-6(3,4) E t=1 |
| 337545.987 | CH3OH 7(5,2)-6(5,1) A-- t=1 |
| 337545.987 | CH3OH 7(5,3)-6(5,2) A++ t=1 |
| 337580.162 | 34SO N,J=8,8-7,7 |
| 337581.604 | CH3OH 7(4,4)-6(4,3) E t=1 |
| 337589.900 | HCOOH 15(6,* )-14(6,*) |
| 337605.276 | CH3OH 7(-2,5)-6(-2,4) E t=1 |
| 337610.627 | CH3OH 7(-3,4)-6(-3,3) E t=1 |
| 337625.745 | CH3OH 7(2,5)-6(2,4) A++ t=1 |
| 337635.750 | CH3OH 7(2,6)-6(2,5) A-- t=1 |
| 337642.484 | CH3OH 7(1,7)-6(1,6) E t=1 |
| 337643.921 | CH3OH 7(0,7)-6(0,6) E t=1 |
| 337646.021 | CH3OH 7(-4,3)-6(-4,2) E t=1 |
| 337648.188 | CH3OH 7(-5,3)-6(-5,2) E t=1 |
| 337655.174 | CH3OH 7(3,5)-6(3,4) A++ t=1 |
| 337655.211 | CH3OH 7(3,4)-6(3,3) A-- t=1 |
| 337655.211 | CH3OH 7(4,4)-6(4,3) A-- t=1 |
| 337671.194 | CH3OH 7(2,6)-6(2,5) E t=1 |
| 337685.218 | CH3OH 7(5,2)-6(5,1) E t=1 |
| 337685.594 | CH3OH 7(4,3)-6(4,2) A++ t=1 |
| 337685.594 | CH3OH 7(4,4)-6(4,3) A-- t=1 |
| 337707.520 | CH3OH 7(-1,6)-6(-1,5) E t=1 |
| 337722.348 | CH3OCH3 7(4,4)-6(3,3) EE |
| 337722.995 | CH3OCH3 7(4,4)-6(3,3) AE |
| 337726.967 | t-CH3CH2OH 19(7,12)-19(6,13) |
| 337730.742 | CH3OCH3 7(4,4)-6(3,3) AA |
| 337731.850 | CH3OCH3 7(4,3)-6(3,3) EA |
| 337732.186 | CH3OCH3 7(4,3)-6(3,3) EE |
| 337748.771 | CH3OH 7(0,7)-6(0,6) A++ t=1 |
| 337770.614 | CH3OCH3 7(4,4)-6(3,4) EA |
| 337778.025 | CH3OCH3 7(4,4)-6(3,4) EE |
| 337779.470 | CH3OCH3 7(4,3)-6(3,4) AE |
| 337785.100 | HCOOH 15(5,11)-14(5,10) |
| 337787.216 | CH3OCH3 7(4,3)-6(3,4) AA |
| 337787.800 | HCOOH 15(5,10)-14(5,9) |
| 337787.863 | CH3OCH3 7(4,3)-6(3,4) EE |
| 337790.076 | CH3OCH3 7(4,3)-6(3,4) EA |
| 337811.639 | CH3OCHO 44(6,39)-44(5,40) A |
| 337825.275 | HCCCN 37-36 1v7 =1f |
| 337829.100 | unidentified |
| 337839.300 | unidentified |
| 337844.400 | unidentified |
| 337877.500 | CH3OH 7(1,6)-6(1,5) A t=2 |
| 337892.524 | SO2 21(2,20)-21(1,21) 1v2 |
| 337938.089 | CH3OH 20(-6,14)-21(-5,16) E |
| 337969.434 | CH3OH 7(1,6)-6(1,5) A-- t=1 |
| 337969.600 | unidentified |
| 337973.000 | unidentified |
uv sampling
| FieldID | SpwID | uv_min(klambda) | uv_max(klambda) |
|---|---|---|---|
| 0 | 0 | 13.298 | 131.839 |
| 0 | 1 | 13.298 | 131.839 |
| 0 | 2 | 13.298 | 131.839 |
| 0 | 3 | 13.298 | 131.839 |
| 2 | 0 | 15.388 | 124.683 |
| 2 | 1 | 15.388 | 124.683 |
| 2 | 2 | 15.388 | 124.683 |
| 2 | 3 | 15.388 | 124.683 |
| 3 | 0 | 16.581 | 118.593 |
| 3 | 1 | 16.581 | 118.593 |
| 3 | 2 | 16.581 | 118.589 |
| 3 | 3 | 16.581 | 118.588 |
| 4 | 0 | 16.646 | 124.602 |
| 4 | 1 | 16.647 | 124.602 |
| 4 | 2 | 16.647 | 124.602 |
| 4 | 3 | 16.646 | 124.602 |